A Landscape of Fear of Humans
By Asia Murphy
Animals, even apex predators, take great pains to avoid people—a pervasive problem when these changes disrupt what they eat and where they go.
Animals, even apex predators, take great pains to avoid people—a pervasive problem when these changes disrupt what they eat and where they go.

With just one video from a remote trail camera, ecologist Justine Smith, now a professor at the University of California, Davis, evocatively showed the fear that one clawless, fangless, poisonless species can strike in the heart of an apex predator.
On a cold, rainy night in March 2015, a trail camera Smith had installed recorded a puma in the Santa Cruz Mountains. (See the sequence of stills below.) As the video begins, the sound of frogs can be heard, and then a puma, glancing carefully over its shoulder, emerges from the brush. The words “Frog (control) playback” are emblazoned on the bottom of the video. As the leaves in the background dance to the tip-tap of the rain, the puma approaches an undistinguished mass that could be mud but is meat. Perhaps it’s the carcass of an unfortunate mule deer, now reduced to its trunk by periodic feedings and decomposition. The apex predator’s large paws hold one end of the mass as it tears into flesh and fur. The video fades to black and the curtain descends on what can be thought of as Act I of this 15-second play.

Santa Cruz Puma Project / youtu.be/TVqOhkK8fKk
The intermission passes in the blink of an eye. The sound and image of the rain-spattered puma, crouching again at its meal, returns for Act II. As the words “Human playback on” appear on the bottom of the video, the peaceful scene is interrupted by the voice of an older man talking about, of all things, a court case. A quick glance toward the direction of the sound—a reflex, really—is all the puma gives. Its body and brain have already decided on another course of action. Despite there being no actual human sharing its little shrubby enclosure, likely no human within a mile of it on such a night, the large cat leaps away, sliding under a log, disappearing almost soundlessly.
Exit, pursued by a human voice.
Wild animals, even the apex predators that we fear, are terrified of us. Smith didn’t just find that the puma was afraid; she showed that pumas feed far less when they hear human voices compared to frogs. Ecologists have spent decades focused on the influence of fear on the relationships between predators and prey, and between apex predators and the smaller predators that they sometimes kill. But in recent years, the focus has shifted. We have begun to look at how animals’ fear of humans influences a variety of phenomena, ranging from predation rates to ecosystem restoration. In the increasingly human-dominated world, successful wildlife conservation and management must take into account this fear—which has long gone unaccounted for.
Although biologists knew that animals were afraid of being eaten—and even studied how vigilant prey animals were and how they hid in groups—it wasn’t until the 1980s and 1990s that we began to compile data that showed that animals are aware of their risk of becoming prey (their so-called predation risk). Not only are they aware, but they actively work to decrease that risk, resulting in a cascade of effects that are equal to or even greater than the effect of a predator simply eating an animal. In 1999, Joel S. Brown and his colleagues at the University of Illinois, Chicago, gave the burgeoning field its title: the ecology of fear.

Nayan Khanolkar / Nature Picture Library
The idea that an organism might be afraid of being eaten is intuitive, but conceptualizing fear ecology allowed researchers to follow that intuition to its logical—or even surprising—conclusions. Imagine the following series of events: To avoid being eaten by a puma, a mule deer might avoid the forest interior for an open meadow, where an ambush predator would find it hard to hide. If enough mule deer avoid the forest interior for the safety of an open meadow, they will eat more plants in the open meadow than the forest, which would change the vegetation structure of both areas. Wildflowers that native pollinators require might decline in the meadows, reducing the numbers of insects that sustain frogs and songbirds, in turn reducing the predators that eat them. The forest interiors might become too overgrown, pushing out birds that require open understory, such as ovenbirds; but the lack of mule deer might also allow oak seedlings to grow to maturity, regenerating the forest canopy. Due to the lack of wildflowers in the open meadows—wildflowers that were perhaps the star attraction for a national park—tourists might stop coming, taking their vital admission fees with them.
In the decade following Brown’s foundational work, a flurry of papers came out, bolstered by the reintroduction of wolves into Yellowstone National Park. Yellowstone was not predator-free prior to wolf reintroduction, but there were ecologically important differences between those other predators and wolves. Coyotes are not large enough to kill elk and bison, and although pumas are large enough to kill elk on occasion, the large ungulates could easily avoid pumas by choosing to browse in open habitats—such as delicate riparian areas—that would stymie an ambush predator. Wolves, however, are not ambush predators. They chase down their prey, and need open habitats to do so. Wolves are also large enough—and numerous enough, compared to the solitary puma—to kill adult elk and bison, allowing them to regulate ungulate populations more effectively. Iconic studies by William J. Ripple and colleagues of Oregon State University found that the mere presence of wolves scared bison and elk into avoiding riparian areas, allowing overbrowsed species such as willows and aspen to regenerate (although a competing hypothesis claims the simultaneous rise in the local beaver population was the cause).
It wasn’t only wolves that were striking fear into the hearts of prey animals, and it wasn’t only in Yellowstone. Studies show that kangaroo rats in New Mexico avoided searching for food on particularly moonlit nights, terrified of death from above in the form of ghostly owls. Kenyan zebras that avoided woodland patches during the day—when lions used them—rested in them at night. Tiny silver fish in dark Norwegian waters changed the depth at which they schooled with the lengthening of the days to stay safely hidden from larger fish. Due to how tightly tied each individual is to another in the web of life, changes in the behavior of one species influence the behavior of another, influencing the behavior of another, on and on, spreading out like ripples on a still pond. Because fear works on a moment-to-moment basis, it is infinitely more reactive when compared to slower evolutionary processes. The same kangaroo rats that remain in their burrows one night for fear of the illuminating moonlight will spend hours foraging freely the next if clouds happen to extinguish the light that makes them visible to predators.
It didn’t take long before the ecology of fear was applied not only to predator–prey relationships, but to predator–predator relationships as well. Smaller carnivores, such as swift foxes, African wild dogs, and European genets, appeared to change the habitats they used or when they were active when larger carnivores that would kill them—coyotes, lions, and lynx—were around. The Yellowstone wolves didn’t just regulate elk and bison populations to healthy levels and potentially help save riparian areas. When Yellowstone had lacked wolves, coyotes had been free to kill pronghorn, the Western Hemisphere’s fastest land mammal and the only survivor of a giraffe-adjacent ungulate family that went extinct in the Pleistocene. A study by Kim M. Berger and colleagues at Utah State University found that by both killing coyotes and making them avoid certain areas, wolves regulated coyote numbers and predation on pronghorns, allowing their populations to rebound.
Fear is the reason an animal that is active during the day might suddenly change to being only active at night. Fear is the reason that an animal that is typically solitary might decide to buddy up with a few pals. Fear is the reason an animal might pass by a forest patch rich with food for one where food is scarcer, but where things are safer. Fear is the background hum of continuous, sometimes intensifying, stress that forces tadpoles to limit their size in exchange for better evasion ability, that makes eider duck eggs less likely to hatch, that makes grasshoppers in cages with spiders that have their mouthparts glued shut just as likely to die as those in cages with spiders that are free to eat them. Fear runs up the trophic food chain, stopping at the sharp teeth or claws of whatever predator is the most dominant, the apex of the ecological pyramid. Everybody is afraid of the big bad wolf, but the big bad wolf fears no one.
Or so we thought. A flurry of research over the past decade has shown otherwise.
Animals, even large predators, have good reason to fear humans. Apex predators have always been hunted around the globe—fearfully, vengefully, ceremoniously. The hunting of apex predators, however, became a concentrated widespread extermination during European imperialism. By the 1600s, the largest carnivores had been hunted out of most of Europe, so it was with some trepidation that the colonizers found that North America was home to pumas, grizzly bears, wolves, and, rarely, jaguars.
“The whole continent was one continued dismal wilderness, the haunt of wolves and bears and more savage men,” John Adams wrote in his journal in 1756. Wolves and bears and pumas were dangers, not only to their lives, but to their livelihoods as ranchers and their recreation as hunters. Manifest destiny had decreed that it was humankind’s responsibility to “tame” North America’s uncivilized wilderness. “Large predatory animals, destructive to livestock and to game, no longer have a place in our advancing civilization,” said E. A. Goldman of the U.S. Biological Survey in 1925. And so European settlers went to work, destroying the forested refuges of these large and dangerous pests, shooting and poisoning and snaring whatever apex predator they could catch. They did such a good job that the places apex predators could be found contracted by almost half, according to a formative 2004 study by Andrea S. Laliberte of New Mexico State University and Ripple. (See “America’s Cat Is on the Comeback,” November–December 2018.)

© Musée de Picardie, Amiens / Bridgeman Images
This extermination of large predators was not only limited to the United States. Fifty times the current global population of tigers were killed in a 50-year period in India during British colonialism, according to environmental historian Mahesh Rangarajan. The thylacine, a carnivorous marsupial, was hunted to extinction in Australia, Tasmania, and New Guinea by the early 1900s. Using DNA from museum and current-day specimens, Simon G. Dures and colleagues of the Zoological Society of London found that lion populations in Botswana, Namibia, Zambia, and Zimbabwe declined suddenly and rapidly, causing a population bottleneck, beginning in the late 1800s during British and Germanic colonization. On average, 17 species of apex predators across the globe now occupy less than 50 percent of their historical range.
Whether apex predators retain a genetic memory of this extermination or are reacting to current-day persecution, the effect is the same: Apex predators across the world are afraid of humans. And this fear causes cascading effects.

Rolf Nussbaumer Photography/Alamy Stock Photo
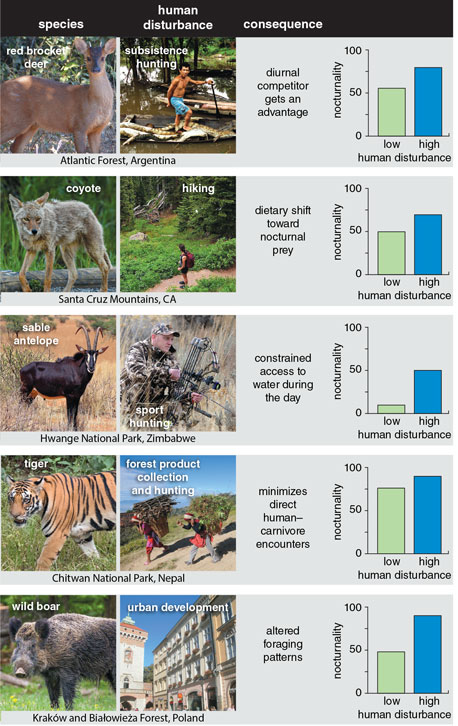
Today, in Switzerland, Eurasian lynx hunt prey near villages, but only after dark when people are asleep. In similar ecosystems across Europe, roe deer become more diurnal to avoid the now-nocturnal lynx’s predation; however, this behavior puts them in the crosshairs of human hunters, who are active during the day. After aerial culling, wolves in Alberta have become nocturnal, decoupling from their diurnal white-tailed deer prey in such a way that it might allow the deer to invade more of Alberta’s ecosystems than they already have, potentially putting already threatened woodland caribou under intense competitive pressure.
Ungulates such as white-tailed deer and moose will “shield” their offspring by giving birth close to houses and villages, using the local apex predator’s fear of humans against them to provide a safe environment for their offspring to grow up. Californian pumas, unwilling to spend too much time at one kill for fear of being stumbled upon by a human, kill more deer in urban areas than they would under less stressful circumstances. Despite this increased pressure on their populations, however, deer, by choosing to eat closer to houses and neighborhoods to avoid pumas, are encouraging these habitat edges to become thicker, bushier, and harder to move through.
Okay, you might say. We are disrupting the lives and interactions of animals and plants, just by being there, but that’s only in the city. We have protected areas, nature reserves, and national parks where apex predators must surely be free of fear and thus able to act without the looming fear of humans. To that, I’ll point to studies that show that human recreation—things as innocuous as biking, skiing, or even hiking—is still enough to cause fear-induced behavioral changes in wildlife. Shalene L. George of the University of Wisconsin and Kevin R. Crooks of Colorado State University found that the presence of hikers and bikers in a natural reserve system in California made mule deer one-third less likely to be active during the day. Jesse S. Lewis of Arizona State University and his colleagues found that bobcats in Colorado were less likely to appear at locations with many hikers. Even grizzly bears in the Rocky Mountains of Alberta were found to avoid hikers and off-roaders, and females with cubs moved three times more than usual in response to motorized recreational activity.
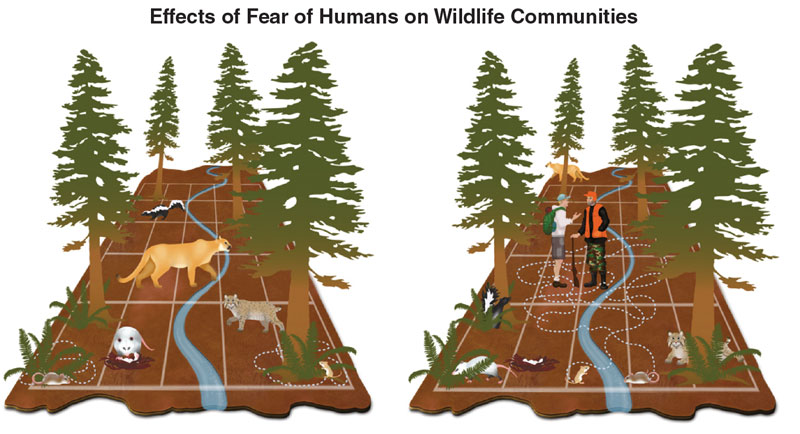
Illustration by Corlis Schneider from Suraci et al. 2019.
During the Anthropocene, humans are taking more than their share, not just of material resources, but also space and time. This infringement squeezes wildlife into ever smaller slices of the landscape and the day, increasing the likelihood that species that only coexist thanks to careful partitioning of time and space will be forced to interact. Multiple studies have found that spatial overlap between different species increases in habitats that get more human visitation. Travis Gallo and colleagues found that cottontail rabbits didn’t spatially avoid coyotes in Chicago urban areas. My own research in Pennsylvania showed that, through avoiding humans, white-tailed deer fawns were forced into being active at the same times of day as black bears and bobcats, both carnivores that love eating fawns. This concurrent activity increases the fawns’ chances of running into their predators and being eaten. A 2018 meta-analysis in Science documented that 62 species ranging from wild boar to tigers increased their nighttime activity by an average factor of 1.36 in response to a variety of human activities, such as hunting and hiking. Conversion of habitats into agricultural land and urban developments also increased wildlife use of the nighttime, once again forcing species that might otherwise use different times of the day to avoid each other into concurrent activity, increasing their likelihood of interacting.
The ecology of fear can be used for good, particularly in human-wildlife conflict situations centered on livestock. In addition to the ever-handy scarecrow, a 2003 study in Idaho using carcasses as an animal attractant found that motion-activated speakers that played loud sounds, including people yelling, reduced carcass-consumption by wolves, black bears, and red foxes by 68 percent.
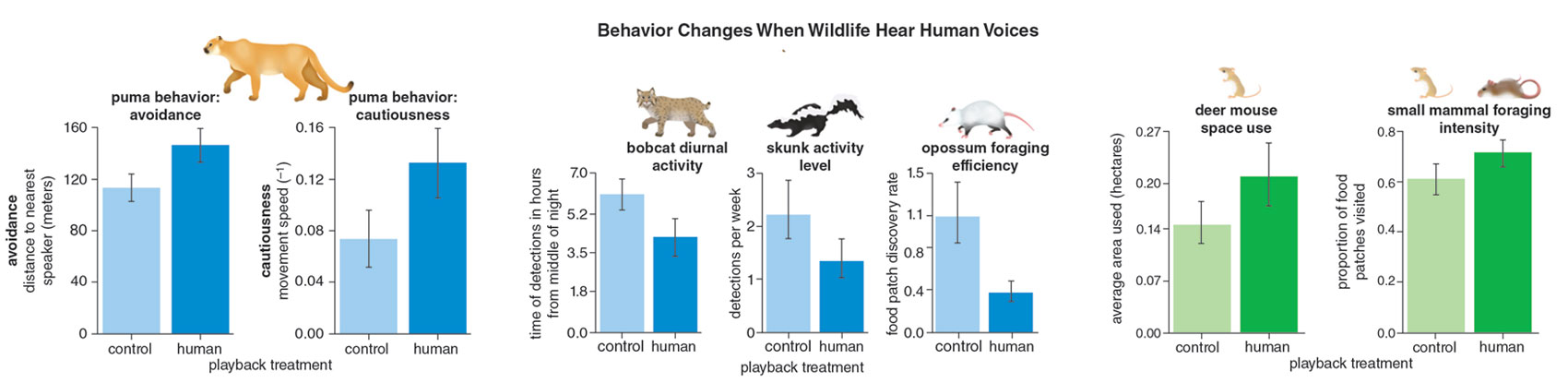
Illustration by Corlis Schneider adapted from Suraci et al. 2019.
On the other hand, as anyone who regularly observes animal behavior knows, animals are smart and often become habituated to humans. The urban coyotes of Chicago and San Francisco come to mind. (See “Suburban Stalkers: The Near-Wild Lions in Our Midst,” September–October 2017.) Still, despite these animals’ abilities to live around us, our urban environments might continue to be more stressful for wildlife than natural ones, as Julia L. Nelson of the University of California, Stanislaus, and her colleagues found in San Joaquin kit foxes. In addition, although animals might adapt to the Anthropocene, the continued background hum of stress presented by humans and our cities and suburbs might dull their predator-avoidance reflexes, as predicted by the risk allocation hypothesis.
In 1991, Steven L. Lima of Indiana State University and Peter A. Bednekoff of the University of Michigan broke down how animals should respond to low-risk and high-risk situations. They hypothesized that temporal variation in risk is key to that response: Animals that are faced with brief, infrequent high-risk situations should respond in a more intense way compared with animals that are constantly in high-risk situations.
My research in Pennsylvania also supports this risk-allocation hypothesis. I found that white-tailed deer vigilance behavior increased with predator relative abundance, but only in the state forest that was surrounded by more forest. In the state forests surrounded by agriculture and urban development, there was no relationship between predator relative abundance and deer vigilance. In addition, deer vigilance in the same state forests was highest during the day, when humans were more likely to be around. This result suggested two things: Deer are more afraid of humans than their natural predators, and something about the more anthropogenically disturbed environment created such a chronic high-risk situation that deer vigilance was decoupled from predator relative abundance. This decoupling could make it easier for predators to kill fawns, potentially influencing deer population density.
Wikimedia; Luiz Claudio Marigo/Bluegreen Pictures/Alamy Stock Photo; National Park Service ; Wikimedia; Wikimedia; Wikimedia; Wikimedia; Bikas Rauniar / Department for International Development; Wikimedia; Wikimedia; adapted from Gaynor et al. 2018
There will always be animals that are fearful of humans. What can we do for them? Imagine a more charitable world where we respect our fellow passengers on this tiny blue rock. National parks and other protected areas could limit the number of daily visitors and completely close off certain sections during particularly sensitive biological periods, such as when offspring are born. We might design greenways with more buffer habitat than we do currently, to give wildlife more space in urban areas. We could plan our cities to reduce suburban sprawl and turn off external lights to erase as much of our presence at night as possible. Wild animals react to variations in human presence: Deer and wild boar activity patterns and movement rates have been found to change depending on whether it was hunting season or not, and bats were found to be less active in Greensboro, North Carolina, during the weekend, when more people were out and about. By understanding the fear that we instill in wildlife, and by being respectful, considerate planet-mates, we can better coexist with other species.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.