The Chemical History of Superior Glass
By Ainissa Ramirez
A glassmaker and a physicist teamed up to improve scientific lenses. Their developments revolutionized both laboratory equipment and domestic cookware.
A glassmaker and a physicist teamed up to improve scientific lenses. Their developments revolutionized both laboratory equipment and domestic cookware.

Otto Schott had dreams of discovering new things in the tidy and clean space of a chemical laboratory. Unfortunately, he was born into a family of glassmakers in Witten, Germany, in 1851, who worked in the heat, sweat, and dust of workshops.
For generations, both sides of his family toiled in this strenuous and stagnant trade, and there was the expectation—spoken and unspoken—that he would join his father in the windowpane factory. Young Schott had other plans. He took every chemistry class he could, starting from high school, to prepare for a doctorate in organic chemistry. Schott, a short and slight man with a handlebar mustache, wanted to leave his mark by using his brain to understand materials, not his brawn to fashion them.
Chemistry in the 1870s was the route to many exciting innovations, particularly in the making of drugs, fertilizers, and explosives. Organic chemists were enchanted with the ability to copy substances made in nature, such as the flavor of vanilla, and artificially duplicate them in the laboratory. Nature did not give up her secrets easily, but when her methods were decoded, these molecules became new products, manufactured by the ton. The world was smitten with what organic chemists could do.
With visions of molecules dancing in his head, Schott applied for a graduate research position at the University of Leipzig. There was no space for him, though. Disappointed but undeterred, he tried to enter organic chemistry sideways, by taking graduate classes in agricultural chemistry. But he soon found this new topic uninteresting and dropped out. With his dream thwarted, he returned to glass, but this time for his doctoral studies, completing them in 1875 at the University of Jena. The title of Schott’s dissertation was “Contributions to the Theory and Practice of Glass Fabrication,” a subject he knew well from the time he was a boy.

INTERFOTO/Alamy Stock Photo; World History Archive/Alamy Stock Photo; The Corning Museum of Glass courtesy of John Littleton
After his graduate studies, Schott went on to work in a glass factory, publishing papers on the melting of glass, the strengthening of glass, and the chemical elements in glass. Schott returned to his hometown of Witten in 1878, steadily experimenting in glass on the factory floor. His work did not set the world on fire, but by using fire and chemical ingredients he hoped to unlock the workings of this old material and make it new.
About 400 kilometers west from a wistful Schott was a disgruntled Ernst Abbe in a laboratory in the college town of Jena. An esteemed professor of physics and the director of a telescope observatory, Abbe had grown frustrated with the glass lenses in his microscopes and telescopes. The professor, with his mathematician’s unkemptness of finger-combed hair, a scruffy graying beard, and spectacles perched at the end of his nose, noticed there were multitudes of flaws in his scientific lenses, making it difficult to see anything clearly. Sometimes the glass had bubbles, streaks, or lines—called straie—that looked like a ship’s narrow wake. Sometimes the glass was cloudy, hazy, or swirled, with unblended parts like a marble cake. Above all, the glass was faulty because colors, such as blue and red, separated within an image, as if seen through modern 3D glasses. With such horrible materials, the chance of scientific breakthroughs was nearly impossible. Without good glass, science was blind.
To vent his frustration about the lack of research on glass, Abbe did what any man of science would do. He wrote a report in 1876 stating that the future of the tweed-wearing scientists’ fine optical instruments such as microscopes and telescopes were in the thick and calloused hands of apron-wearing glassmakers.
The earliest glasses entailed heating and mixing the ingredients of sodium carbonate (or soda), limestone (chalk), and silica (sand), creating crown glass, which was used for windowpanes and bottles. Replacing the chalk with lead compounds created the more ornate flint glass, also called lead crystal. These two families of glasses had been the only ones for centuries, and Abbe declared there was a scarcity of studies that explored new additives to make glass with improved optical properties.
In his report, Abbe set a new research direction, stating that “the development of new types of optical glass with uniform, calculable, and predictable properties was required.” Abbe wanted the manner of how a glass interacts with light to be cooked into it. Just as a baker changes the amount of flour, water, and yeast to modify a bread’s texture and chewiness, Abbe wanted to know how the chemical ingredients changed the glass’s ability to fan out white light into colors of the rainbow, or its ability to bend light, the way it makes a straw seem broken in a beverage. Abbe wanted these traits to be turned up or down in a regular and repeatable way, with the knob being the chemical elements that make up the glass. His report went on to say how little had been done in the study of glass for decades, and he stated openly what many knew but were too polite to mention, particularly that the making of glass was based on traditional recipes and not on technical know-how. And without that know-how, science was going nowhere.
Schott got hold of this report three years later, and wrote a letter to the professor in 1879 volunteering to supply a range of different types of glasses, with the hopes of escaping the sweltering heat and silt of the factory floor. Schott had been working on systematically creating glasses of different chemical ingredients, but he didn’t have access to a laboratory to do the scientific measurements to see what his glasses could make. Abbe had access to those instruments, but didn’t have the ability to make new glasses. Together, these men were each other’s yin and yang. Abbe was willing to collaborate with a person unknown in the sciences, because he had nothing to lose. Schott was keen on doing extra work, because he had everything to gain. This opportunity was Schott’s shot.
Schott sent glass samples to Abbe, but they did not have the optical properties that Abbe desired. Nevertheless, the two struck up a correspondence, and Schott continued to make glasses of different combinations of chemicals. He was able to make better choices than scientists of the past because 20 years earlier a Siberian scientist, Dmitri Mendeleev, had turned the field of chemistry upside down with his breakthrough of the periodic table, in which all of the known ingredients of the world—the elements—were found to systematically relate to each other on a chart, where elements near each other on this chart acted like cousins. (See “The Grammar of the Elements,” November–December 2019.) Using the new periodic table, Schott began to take a methodical approach to exploring how different concoctions of glasses behaved. New formulations under the guidance of this table allowed Schott to make better educated guesses.
Schott set plans in 1880 to make new glass mixes, treating the periodic table like a restaurant menu, selecting options from different columns, and sometimes from the same column, to see what worked best. He began his efforts by adding the elements of phosphorus and boron. In the fall of 1881 he focused on boron, which comes from borax (a detergent additive), and discovered something very promising: Adding the related compound boric acid made a new type of glass, borosilicate glass, which seemed free from defects. Schott sent these new glasses to Abbe for testing, looking forward to the results. In a letter dated October 7, 1881, Abbe wrote, “The problem [of flaws in optical glass] has been solved.” The professor followed up on that note by inviting Schott to visit Jena to demonstrate his new glass.

Ainissa Ramirez
With continual improvements over the next year, Schott got his secret wish. Abbe wrote that he thought it best for Schott not to continue his work in a glass factory, but insisted that it be done in a chemical laboratory in Jena. Schott made arrangements to leave Witten.
In 1882, Schott moved to Jena to run a small-scale operation in partnership with Abbe and microscope-maker Carl Zeiss, with whom Abbe had a long-standing business relationship. No longer were Schott’s experiments confined to small furnaces where he could create samples no bigger than a cup of sugar. His specimens were now huge icicles the diameter of bowling balls. Schott started a company in 1884, Schott & Associates Glass Technology Laboratory, to make and sell specialized glass. Their first catalog, published by the company in 1886, had 44 different glasses; by 1892, there were 76.
Schott devised new formulations for better optical lenses, and later for thermometers, too. In the late 1800s, thermometers were one of the few tools that scientists had to probe a chemical reaction. At the time, chemistry was limited to knowing how hot things got (the temperature), how much they weighed (the mass), how much space they took up (the volume), and how much they pushed the container walls (the pressure). Many scientists noticed that their temperature readings were higher than they should be. It turned out that thermometers did not return to the proper baseline once they were cooled. The repeated heating and cooling that the thermometers underwent modified the glass so that the bulb, which held the mercury, changed its shape, causing the mercury to creep up. This meant that subsequent temperature readings were not to be trusted. By tailoring the amount of boron, Schott was able to build a glass that did not adjust its shape when heated, allowing thermometers to make proper readings.
Schott cranked out different flavors of glasses in collaboration with Abbe. One was the glass for thermometers that did not alter its form with heat. Another was optically superior and perfect for scientific telescopes and microscopes. A final one did not dissolve in water, acid, or other liquids, making it suitable for laboratory experiments. At the heart of his new creations was boron, though boron played different roles in each of Schott’s new glasses.
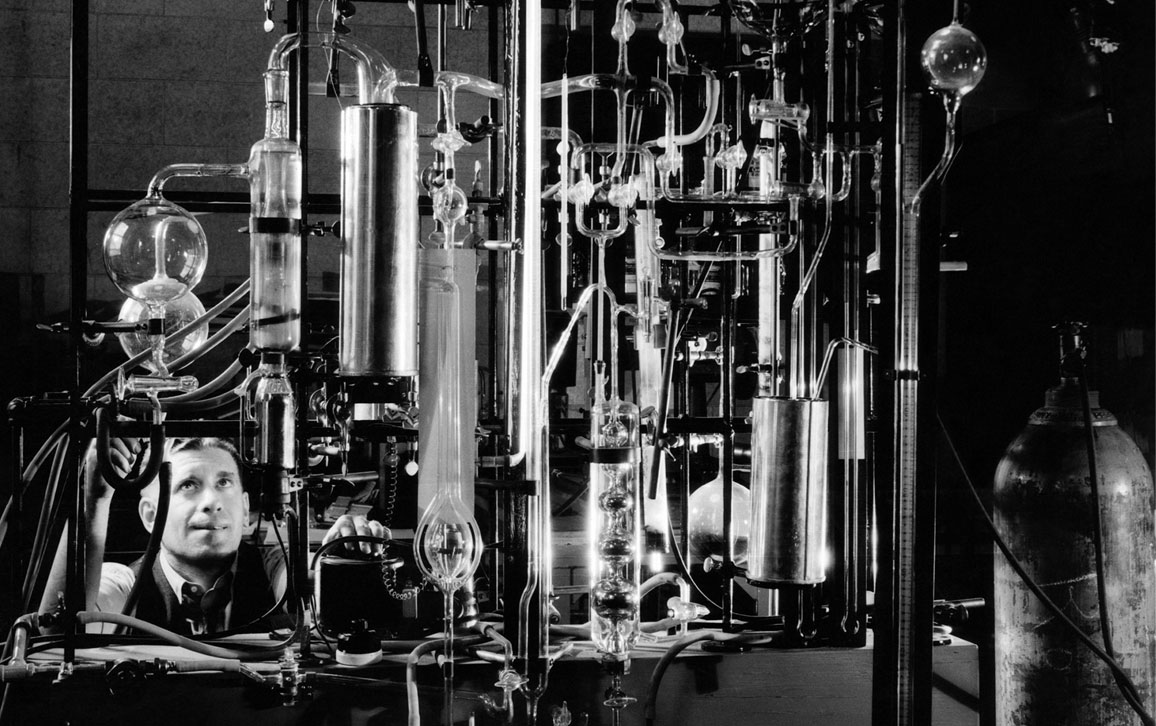
Everett Collection Historical/Alamy Stock Photo
Schott’s glasses had versions with small, medium, and large amounts of boron, the way a chef can make a sauce with different levels of spice—mild, medium, or hot—based on the amount of pepper in it. For glasses with improved optical properties, a small bit of boron was added to windowpane glass, giving it a better ability to bend light. For glasses that did not expand when heated, Schott added lots of boron. Boron tightly grips other atoms with stiff bonds, like a strong spring, causing the resulting glass to resist expanding when it gets hot. Finally, for glasses capable of withstanding dangerous chemicals such as acids, the amount of boron was decreased to a medium level. Boron likes to bond with other atoms, but its bonds are weak in acids. So some of the boron was taken out of harm’s way and substituted with other compounds. Together, all these ingredients stabilized the glass in these harsh environments.
Soon, the glasses designed by Schott became the most desired scientific glass on the planet, and Germany became the main source of all glasses for microscopes, telescopes, and laboratory ware (beakers, flasks, and test tubes). Every scientist wanted optical instruments with the name “Jena” inscribed on them. For other glassmakers, penetrating this glass market seemed impossible.
In the early 1900s, American glassmakers wanted to develop an alternative to Germany’s Jena glass. Cracking the code for Jena borosilicate glass wasn’t easy, though. Glassmakers knew that boron was a key ingredient, but the rest of the recipe was a mystery. Schott spelled out in his highly technical papers the factors that allowed glass to withstand high heat and large temperature differences, but few glassworkers could translate the theory into practice on the factory floor. To be successful, one American company, Corning Glass Works of Corning, New York, knew that their workmen were going to need some help from academics.
The glass was inferior, and with such horrible materials, the chance of scientific breakthroughs was nearly impossible. Without good glass, science was blind.
Corning Glass Works was a family-run company that mostly made decorative glass and tableware, and soon also handblown glass for Thomas Edison’s light bulbs. If they were going to compete with Jena glass, though, they knew they needed better science behind their processes. Corning started to move away from using glass recipes passed down from one generation to the next and began to apply the scientific method. One of the first things Corning’s management did was tell their workers to write down what they added to a glass melt, so that a batch could be replicated if need be.
Starting in 1908, chemists were on Corning’s payroll, and the investment was working out to be a wise one. The scientists knew that boron was a key ingredient in these new glasses, and eventually, by trial and error, they created a version of borosilicate glass called Nonex (short for NON-EXpanding glass). Unfortunately, Corning still could not penetrate the labware market with it. Nonex was no rival to Jena glass, which had a nearly 15-year head start. Additionally, German glass enjoyed low tariffs, since it was an educational product. Customers saw no reason to buy American-made glass when the price of the superior glasses from Germany was not prohibitive. Corning’s management had to find a domestic market for their borosilicate glass and reached out to the most lucrative industry in the nation to help keep the company afloat: the railroad.
In the early 1900s, the tentacles of railroads reached some of the farthest corners of the country. The railroad compressed space and time with its speed. That speed came at a cost, however. As trains became faster, many more catastrophic accidents and collisions occurred, and with that came a need for better signaling to increase safety. Signals on the tracks told trains not to proceed, with warnings from hot arc lights with red glass covers. On rainy or snowy days, however, accidents occurred more frequently. In addition to the inclement weather, another cause for the increase in accidents was the frailty of glass.
Glasses on train signals were between a rock and a hard place on days with bad weather. The interior of the glass signal was heated by the hot arc light, causing it to expand; the outside, however, was dramatically cooled by the rain or snow, causing it to shrink. The conflicting messages within the glass gave rise to pent-up stress; when prolonged, this stress resulted in broken glass. A red glass alerted the train to stop, but a broken glass was no longer red, which gave a false message that it was safe to pass, potentially causing a colossal collision. And as if the weather was not enough for the glass to contend with, mischievous boys used train signals for BB gun target practice, smashing the red glass into pieces with a single pellet. The railroads needed better glass to mitigate the weather and the delinquents, and Corning’s strong Nonex glass did the trick.
Corning’s glass rarely failed; however, the company soon became a victim of its own success. When the railroad adopted Corning’s glass, there was a boom in sales, but the indestructibility of the glass meant that once the railroads purchased their hardy glass, they didn’t need replacements. The meteoric increase in sales was followed by a precipitous drop. This lack of built-in obsolescence, or a limitation that would have required additional sales, caused the company to scramble for new glass markets. Help would come from, of all places, a cake.
One summer afternoon in 1913, Jesse Talbot Littleton, a physicist and one of Corning Glass Works’s newest scientists, came to work with a sponge cake baked by his wife, Bessie. J. T., as Jesse preferred to be called, and Bessie were southerners; he was from Alabama and she was from Mississippi. They had moved a year prior to Corning from Ann Arbor, Michigan, where J. T. had been a physics professor, and, together, they were trying to get accustomed to their new Yankee home. In a spirit of Southern hospitality, Littleton brought in the cake.

B Christopher/Alamy Stock Photo
The cake was not just a social offering, though; it was also a science experiment. For the past two weeks, Littleton had been trying to convince his colleagues of the benefits of cooking with a glass container, but they laughed at the notion. For generations, people had been told to keep glass away from heat, so baking with glass seemed ridiculous. Little did they know that Littleton was not only a Southern boy, but also a glass man.
Littleton was obsessed with glass. He talked about glass at the dinner table. When Jell-O came for dessert, he’d break it apart slowly and show his children how it broke like glass. He even had hopes of being buried in a glass coffin. He did his 1911 dissertation at the University of Wisconsin on the heating properties of glass. The rest of the Corning scientists, who were chemists, assumed that the thick walls of the glass would prevent food from cooking evenly and that the heat would not spread as well as it would with a thin metal pan. Littleton, a physicist, knew otherwise. When his colleagues did not listen to his words, he decided to follow them up with action. And he got some help from Bessie.
Bessie Littleton liked having company. She was raised on a remote Mississippi plantation where visitors were few. At her new home in upstate New York, she asked J. T. to bring people from work over for dinner. At barely 5 feet tall, with her dark hair in a bouffant style, she was slight, talkative, and dogmatic. With Bessie things had to be just so, and she had very strict rules that J. T. had to follow, including, according to their son, Joseph (and in his words): no lying or liquor; no cigarettes or cigars; no cussin’ or colored people at her table. The long and lanky J. T., with his tall frame, eyeglasses, serious eyes, permanent pout, and understated grace, complied.
One night J. T. brought over a fellow scientist, H. Phelps Gage. When the men talked about glass after dinner, Bessie had a captive audience for something that had been troubling her.
A few days earlier, Bessie’s new Guernsey casserole dish, which she had only used one other time, broke. All night, the men talked about the indestructibility of glass, and she insisted that these smart alecks ought to make cookware that did not break. The next day, J. T. got two cylindrical Nonex battery jars, about as wide as a basketball, cut off the bottom to make round dishes, and brought them home to Bessie.
Bessie didn’t cook. She had servants to do that. As a child in the South, her servants were freed Black slaves who could not escape the grip of the plantation. As an adult in the North, she hired white immigrant girls whose families had come to New York State for work. While Bessie was no master chef, she dominated with her baking. As soon as J. T. gave her an indestructible glass dish, she got to her favorite kitchen task and turned sugar, eggs, flour, butter, milk, vanilla, and baking powder into a sponge cake. After using every bowl and utensil in the kitchen, she poured the batter into her newfangled dish and baked. What emerged from the oven was an evenly browned cake with a color surpassing that provided by her metal dishes.
The next day, J. T. brought the cake to work, and everyone reported that it was good. He then told them that this cake had been baked in glass, causing scientists to scratch their heads, and men in management to rub their chins. Littleton relayed to his colleagues how easy it had been to remove the cake from the smooth glass pan, unlike metal cake pans. His colleagues did not think glass, if it survived, could make a cake as delectable as the one Littleton had brought in, and, in a sense, they ate their words.
They asked that Bessie try out other foods and report how the glass pan worked. So Bessie, as the resident domestic scientist, had a few items cooked, from French fries to steak to cocoa, although she had a penchant for Southern dishes of grits, corn bread, and collard greens. The pan performed well, the food didn’t stick, and the glass pan didn’t retain the flavor of the food the way a metal skillet did.
On hearing about the success of this glass for cooking food, the Corning management saw promise. But they had to make a few changes and learn a few more things. First, the formulation of Nonex had to be altered, since it contained lead. So the scientists made a borosilicate glass without lead for this bakeware. Next, they had to test the strength of the glass, dropping a weight as heavy as a can of soup onto different types of dishes to see how they survived the rigors of a kitchen. Earthenware cracked with a weight dropped at 15 centimeters, and crockery broke at 25 centimeters, but borosilicate glass laughed off the impact, untouched, even when the weight was dropped from waist high. After these impact tests, the team had to figure out how the glass cooked food. Bessie reported that food cooked quicker than in a metal pan, which was the opposite of what they had believed would happen. They got to the bottom of this mystery with an experiment.
A scientist dipped a Nonex pan into a liquid chemical bath full of microscopic bits of silver. The silver settled on the surface, coating the outside with a thin layer, giving it a mirror finish. Then, they baked two cakes: one in a simple Nonex pan and one in the mirrored one. The cake with the silver coating did not cook well. What they learned is that the heat from the oven walls, like the rays of the Sun, transmitted through the clear glass, cooking the cake, whereas the mirrored surface reflected that heat back. A cake in a metal pan gets heated from the hot air in the oven and the heat from the oven rack. The glass, meanwhile, was letting heat into the cake a third way, via invisible rays of heat, the kind of heat that the Sun uses to brown our skin and that helps create the crust of a loaf of bread.

Everett Collection
To commercialize this glass with a new purpose, it needed a name that informed the consumers, mostly women, what this new glass did. The first commercial piece on the market was a pie tin, which was initially called “Py-right.” It was renamed Pyrex in 1915, to relate to the earlier product, Nonex, and to sound more futuristic and medical, like latex or Cutex. Sales of Pyrex were initially flat, but after the company responded to customers’ needs—by reducing the weight of the ovenware, for example—it became a standard item in households. By 1919, over 4.5 million pieces of Pyrex ovenware were sold. To encourage more sales, Corning created many shapes, sizes, and colors so that new pieces were always desirable (learning their lesson from the railroad glass episode), which made Pyrex a standard Christmas gift. Corning still had its eye on glass for labware, however. An opportunity to enter that market would be a gift of war.
In 1915, with United States’ potential entry into World War I, it became clear that the government needed the ability to make glass for military applications. Jena glass was regarded as the best in the world, but imports from Germany were dwindling. American companies, such as Corning Glass Works, had been encouraged years before to create a substitute. These glasses would be used by U.S. soldiers in gun sights and binoculars, by sailors in sextants and periscopes, by airmen in aerial cameras and range finders, by army doctors in thermometers and vials of medicines, and in the laboratory by chemists for the synthesis of explosives. At the precipice of the U.S. entry into the war, Corning had developed a borosilicate glass, although the ideal Jena formulas were still locked up in German patents.
At the precipice of America’s entry into the First World War, Corning had developed a borosilicate glass, although the ideal Jena formulas were still locked up in German patents.
What the U.S. companies may not have known is that laws of peacetime do not hold during war. When the United States entered the war, it confiscated nearly 20,000 German patents as part of its war booty. Impenetrable German monopolies protected by patents, for dyes such as mauve and drugs such as aspirin, were blasted open with one of the United States’ secret weapons: the Trading with the Enemy Act. With it, German science—the science of the enemy—became fair game to Americans and American companies. Buried within those patents were those recipes for specialty glasses.
After the war, Corning introduced a range of new Pyrex products, filling in for the shrinking supplies from Germany. In laboratories, there were Pyrex petri dishes, test tubes, and flasks. In homes, there were Pyrex cooking dishes, oven door windows, and percolator tops. In automobiles, there were Pyrex headlights, battery jars, and pressure gauge covers. The United States had unknowingly entered the Glass Age, whereby Corning created a new U.S. industry of scientific and specialty glass. To keep this comfy cushion without competition for their consumer commodities, Corning pushed for legislation to prevent the influx of German glass into U.S. markets after the war. Huge tariffs were placed on German glass, preventing Germany from monopolizing these markets as they had done in the past.
These actions were out of view for most Americans, including scientists, who used Pyrex glasses to find the causes for diseases in glass petri dishes, and developed drugs to fight them in glass test tubes. What citizens and scientists did not know was that glass also provided containers that cooked up a new narrative of innovation and scientific prowess. There was no doubt that the United States was a science superpower, but what was unknown at the time was that the nation now had the upper hand, particularly in glass, made possible by a curious combination of war and cake.
This excerpt is adapted from The Alchemy of Us: How Humans and Matter Transformed One Another, copyright 2020 Ainissa Ramirez, by permission of MIT Press.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.