Minuscule Speed Traps
By Fenella Saunders
Rotating molecules experience the Doppler effect
Rotating molecules experience the Doppler effect

DOI: 10.1511/2011.91.300
It can be given credit for helping us to grasp the grandeur of the universe, and it can also be blamed for some modern-day unpleasantness. From the redshift of receding galaxies to the radar guns in speed traps, the same fact of physics underlies the outcome. The Doppler effect occurs when an object is moving while simultaneously emitting or reflecting wave signals, such as light or sound. If the object is moving toward a receiver, it catches up with the signal it emits, contracting the distance between wave peaks. The effect is that sound shifts to a higher pitch, or light to a higher frequency (toward the blue end of the visible spectrum). If the object is moving away, sounds are shifted to a lower pitch and light to a longer wavelength, toward red (which is why the light from stars moving away from us in an expanding universe is described as redshifted.)
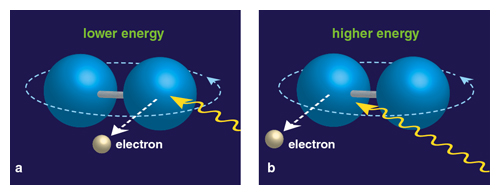
Illustration by Barbara Aulicino.
This effect isn’t restricted to objects moving in straight lines; it also applies if they are turning like a top. This phenomenon is again useful on the astronomical scale, where it is seen in revolving planets or galaxies and is used to determine their rotational speed. But it turns out that this rotational Doppler effect scales down from the most grandiose all the way to the tiniest possible level, at the scale of single molecules. (Atoms are considered points and don’t rotate, so a minimum of at least two atoms joined in a molecule is necessary.) For the first time, this effect in molecules has been experimentally observed, as chemist T. Darrah Thomas of Oregon State University and a team of international colleagues reported in the May 13 issue of Physical Review Letters.
Thomas has suspected for several years that the rotational effect was there in molecules, and he finally got the data to prove it. He and his colleagues were able to show that when electrons are ejected from revolving molecules, the energy of the electron will be slightly higher if it is emitted from the end of the molecule that is rotating toward the receiver, and slightly lower from the end that is rotating away. The effect becomes more pronounced as the temperature increases. “On the astronomical scale, this is old-hat stuff,” says Thomas. “It’s just that it was not seen on the molecular scale for a variety of reasons, one being that nobody had looked for it.”
Thomas and his colleagues conducted their experiments in synchrotrons, particle accelerators that provide a source of light at a wide range of frequencies and energies. The group traveled to three synchrotrons across the globe—in Japan, Sweden and France—to get the span of energies they needed. Their target was a gas that was a mix of solo krypton atoms and nitrogen molecules made of pairs of nitrogen atoms. The team bombarded the gas with photons of various energies from the synchrotron. The photons that collided with the atoms or molecules caused them to emit an electron, and the team was able to measure the energies of the ejected electrons.
“In the ideal world, when you put a photon in at a specific energy required to get an electron out, you would expect to see only a single energy,” explains Thomas. “We don’t, because the photon energy is not precisely defined, and the device used to measure the electron energy distribution is not perfect, so there will be an instrumental energy spread. There’s also translational Doppler broadening because the atoms are moving.” The krypton atoms are in the gas target mix to account for these factors. Both the krypton and the nitrogen molecules will experience the same instrumental effects, and the group can correct for the linear Doppler effect, but only the nitrogen molecules are turning, so only they will have an additional factor of rotational Doppler shift. “When we look at the distribution of electron energies coming from the nitrogen, they are broader than the distributions coming from the krypton,” Thomas says. “By comparing the krypton measurements to the nitrogen measurements, we can figure out the rotational contribution.”
Such effects are important for researchers to account for when they are making measurements of molecules, to make sure that their readings are accurate. This result provides them with a way to increase their precision, but as Thomas notes: “It’s one of these things, be careful what you wish for. We always wish for better resolution, because then life would become simpler. Well life never becomes simpler, it only becomes more complicated, so this is just one more complication that has to go into analyzing and planning the sort of experiments we do.”
Thomas hopes that the data might help to isolate measurements that come from deeper within molecules, such as from electrons that might be ejected from inner shells and only last a fleeting instant. It might also be possible, eventually, to work out whether or not electrons are ejected uniformly in all directions, which might tell something about molecular orbitals. “So there are other things that we might hope to glean, but these are pretty difficult experiments, and getting that kind of information out is a hard struggle, at least at this point,” says Thomas. “It’s probably there, it’s just hard to do. But of course things get better.”
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.