Working Best Under Pressure
By Fenella Saunders
A new type of polymer turns mechanical forces into chemical reactions
A new type of polymer turns mechanical forces into chemical reactions

DOI: 10.1511/2009.79.291
There are plenty of times when physical stress is not a bad thing for natural tissues—in fact, it’s required for their growth. For instance, bone heals best when it is put under a mechanical load. Muscles get stronger when they are subjected to a force. “Hearing is also a mechanically triggered event,” says Nancy Sottos, a materials engineer at the University of Illinois at Urbana-Champaign. “There are many examples that range in scale from protein and cells to the macroscopic level. Biological systems are very successful at using mechanical force to stimulate productive processes that are essential for life.”
In contrast, for synthetic polymers, mechanical stress has invariably led to degradation and eventual failure. But why shouldn’t such materials also respond positively to pressure? “Whenever you apply force to something, it’s doing work,” says Sottos, “and if you’re doing work, that’s energy that is available for a chemical reaction.” Along with a group of chemists and engineers at the University of Illinois Beckman Institute, Sottos is incorporating substances into polymers that can transduce the energy from squashing or pulling into a trigger for a chemical change.
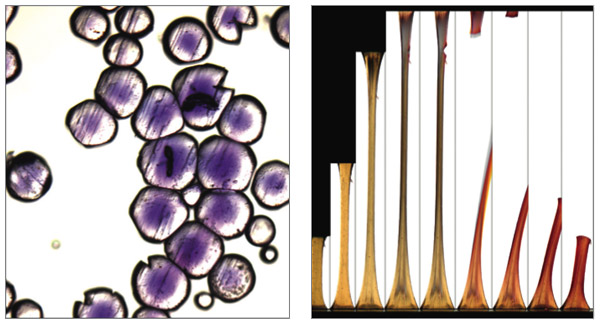
Left image courtesy of B. Beiermann and N. Sottos, University of Illinois Beckman Institute; right image courtesy of D. Stevenson, A. Jerez, A. Hamilton and D. Davis, University of Illinois Beckman Institute.
As a proof of concept, Sottos and her colleagues have developed a polymer that incorporates a type of dye molecule called a spiropyran. When a particular covalent bond in a ring structure of the molecule is broken, the changed shape shifts the molecule’s light-absorption range to longer wavelengths, which gives rise to both visible color (in this case, red) and fluorescence. The group has shown that mechanical force on the polymer can be used to power a chemical response that specifically breaks this covalent bond. The reaction is reversible; both heat and light can reclose the ring after several hours to several weeks of exposure, depending on the stiffness of the polymer.
Experimenting with several configurations, Sottos and her colleagues showed that in a stretchy polymer, in order to transfer the energy from mechanical forces into the chemical reaction that breaks the covalent bond in the spiropyran, the dye molecule had to be in the center of the polymer and experience force from both directions. In glassy polymers, the dye was instead incorporated into strands that cross-linked the long polymer chains. They were also able to prove that the color change was not the result of other factors, such as localized heating when the polymer breaks.
As they reported in the May 7 issue of Nature, the team fashioned two types of experimental polymers. A stretchy polymer, called PMA (for polymethyl acrylate) was molded into a barbell shape, with thicker ends connected by a thinner middle section. When stretched to breaking, the material changed color in the damaged area just before it tore. After rupturing, most of the sample had turned red. The other polymer type was a small, glasslike bead made of PMMA (for polymethyl methacrylate). When the beads were compressed, they also changed color and fluoresced. The team has been able to demonstrate the effect in other polymers, such as polyurethane and polystyrene, so Sottos does not foresee any inherent material limitations for this technique.
Polymers that change color when damaged have obvious use as force sensors that can show when and where a physical fracture has occurred, before there is catastrophic failure. For some applications, the group may need to develop new dyes that are not responsive to light or heat. But the researchers are also developing other mechanophores—chemical groups that are mechanically sensitive. “We’re investigating several new mechanophores that do even more interesting things than change color,” says Sottos. For instance, molecules that cut at specific breaking points when strained could be used as stress-activated fuses. The most ambitious goal of the group is to create polymers that could respond dynamically to stress by locally remodeling, reorganizing or even regenerating.
Self-healing polymers previously have been created that incorporate tiny microspheres filled with repair material; any cracks tear open the spheres, and their contents in turn fill in the cracks. But each capsule can only be used once, limiting the lifetime of self-repair, and some materials don’t work well with the inclusions. “We were making the capsules smaller and smaller, then all of a sudden it dawned on us that maybe we could incorporate something right into the backbone of the polymer,” says Sottos. “We wanted to move to this more seamless molecular approach.”
The material could possibly store spare monomer, which the mechanophore might catalyze to form new polymer. In the most ambitious scenario, such mechanophores might be able to induce new cross-linking of existing, damaged polymer in response to stress, thereby closing a tear. “That’s the ultimate goal,” says Sottos. “I think we have a sound strategy for trying to achieve that. It will not be easy, but I think there will be some interesting progress.”
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.