Spring Budburst in a Changing Climate
By Richard B. Primack, Amanda S. Gallinat
Henry David Thoreau’s 160-year-old field notes document the changing life in the woods, as a warming climate jumbles the timing of annual springtime schedules.
Henry David Thoreau’s 160-year-old field notes document the changing life in the woods, as a warming climate jumbles the timing of annual springtime schedules.

DOI: 10.1511/2016.119.102
As days lengthen and daytime temperatures rise in early spring, forest life wakes up: Buds fatten and then unfurl fresh, green leaves. Birds that wintered elsewhere return and begin courtships. The first frogs begin to call. Spring ephemeral wildflowers emerge among melting patches of snow. Hibernating mammals come out of their dens. The air smells fresher. Every year we look forward to the greening of the forest, to that visceral cue that initiates and coincides with the sudden bustle of spring.

But those of us who study natural history have noticed something troubling: In a warming world, the timing of these events is changing. So, in 2003 we began to study these changes in our research site of Concord, Massachusetts, where the nature writer and philosopher Henry David Thoreau lived in the mid-19th century.
For our first few years studying spring we focused on flowering times and the arrival of birds; we didn’t bother to record when trees put out their first flush of leaves. We thought that something so basic and so environmentally important as the greening of the forest in spring would have been studied long ago. But as we started researching how a warming world was affecting the timing of spring events, we realized how little information existed about what cues new leaves to grow. What we’ve found so far has shown us that the changes are far from trivial. And this realization has led us into a new area of research focused on the timing of leaf events.
Most people recognize intuitively that new leaves emerge in response to spring temperatures—warmth coaxes leaves of trees and shrubs out of their winter buds. Fewer people, though, are aware that warming from anthropogenic climate change is affecting when leaves appear on trees in the spring and when they change color and drop in the fall. Even fewer people appreciate how much these changes are affecting insects, birds, whole ecosystems, and even humans in many important ways.
Every year, leaves absorb a massive amount of carbon dioxide from the air, convert it to carbohydrates through photosynthesis, and transfer it into wood and roots. Seasonal changes in atmospheric CO2 concentrations demonstrate the function and importance of leaves; global CO2 concentrations build up over the winter as leaves and other organic matter decompose, but decline as soon as new leaves emerge in the spring and start their photosynthesis. During this process, leaves also release water into the atmosphere, affecting the local climate and rainfall patterns. They provide food for caterpillars, deer, and many other herbivores, and they provide protective cover for birds, squirrels, and many other wildlife species. All of these processes and interactions begin when leaves first emerge in spring and end when leaves drop in autumn.
Although researchers have been interested in the mechanics of leaves for many decades, the natural history of leaf out and leaf drop was largely ignored. Our interest in leaves began when we saw observations of leaf-out dates buried in Thoreau’s unpublished 1850s field notes. He monitored plants in locations around Concord, including Walden Pond, where he wrote his most famous work, Walden. We wondered if trees leaf out earlier now than they did more than 150 years ago, as the climate has warmed due to increased greenhouse gases and urbanization. When we began the daunting task of cataloguing leaf-out times of the species at Walden Pond, in 2009, we did not expect to see much change in the leaf-out times of trees, because we thought that with their deep roots and large bulk they would be buffered from a warming climate. But after monitoring 43 tree and shrub species for five years in Concord, we have found that these woody plants are now leafing out 18 days earlier on average than those recorded in Thoreau’s observations from the 1850s.
Understanding how leaf-out and leaf-drop times are changing is important for anticipating and preparing for the effects of climate change on forestry, agriculture, gardening, tourism, ecology, and climate. Intrigued by what we found in Concord, we’re now collaborating with several research groups around the world to broaden what we know about leaf emergence. There are still many unknowns about the far-reaching ripple effects of changing spring budburst, but one thing is clear: The repercussions of climate change are upon us. Sometimes we assume that the data we need to make solid predictions already exist, but in our case information on leaf emergence times was surprisingly elusive, sparking new research directions that we like to think would make Thoreau proud.
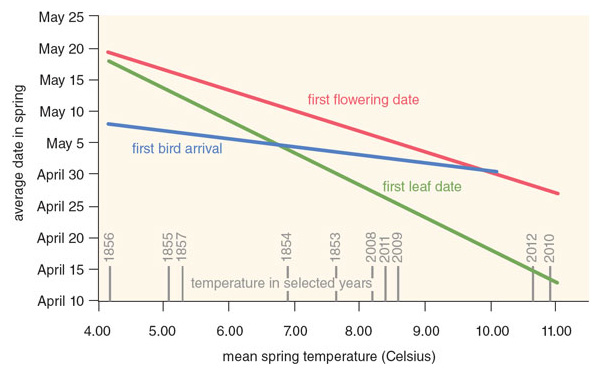
We were extremely fortunate in our choice of locations. The town of Concord possesses one of the best-studied floras in North America, or even the world, and so provides a valuable small-scale test case of a much broader regional and global phenomenon. During his four-hour walks each day around Walden Pond and Concord in the 1850s, Thoreau monitored the leaf-out times of 43 woody plant species—both bushes and deciduous trees—including many common species such as red maple, red oak, and lowbush blueberry. Over the past 160 years, the mean date of leaf emergence has shifted from May 8 in Thoreau’s time to April 20 in recent years.

As we expected, warming spring temperatures have primarily driven this change over time. In Concord, plants leaf out five days earlier for every 1-degree-Celsius increase in average temperature during the spring months of March and April. Plants are now leafing out earlier than in the past, because temperatures in metropolitan Boston have warmed by about 3 degrees Celsius over the past 160 years. Looking at particular species, we have found that woody plants that leaf out earliest in the spring, such as highbush blueberry, are the most sensitive to rising spring temperatures and have shifted their leafing-out time the most; species that leaf out latest, such as silver maple, are the least sensitive and have changed their leafing out time the least.
Eighteen days may not seem like a whole lot, but it can make a big difference for other plants and animals that depend on the appearance of these leaves, and whose schedules may not have shifted in the same way. Eighteen days can mean the difference between a young caterpillar eating tender young leaves or encountering older and tougher leaves, for example. In turn, the health and very survival of those insects are important for birds and other insectivorous animals. If the spring activity of these groups becomes asynchronous, this can limit food availability and lower survival and reproduction for one species or another.
There is huge potential for mismatches to occur among plants, insects, and birds in both spring and autumn, but we cannot predict these changing interactions without first understanding how leaf dynamics are likely to change. For example, certain oaks, such as the white oak (Quercus alba), are changing their leaf-out times due to a warming climate. We need to understand how this earlier leaf-out time affects the numerous insect species that are specialists on feeding on young oak leaves, and in turn on the bird species that feed on these insects. As one specific example, in Europe winter moth caterpillars emerge in spring just in time to feed on young oak leaves and in turn are eaten by migratory pied flycatchers. Now that oak tree leaves and the caterpillars that feed on them are emerging earlier in response to warming, scientists think that the decline of the pied flycatcher may be due to its inability to shift its spring migration fast enough to track the earlier peak of caterpillars.
While our results showed clearly that plants were leafing out earlier in Concord in recent years than in Thoreau’s time, we still did not really understand what drove the differences in emergence times. Why did some species leaf out earlier than others, and why were some species particularly responsive to a warming climate?
To figure out what induces trees to leaf out, we used a simple technique known to many gardeners and plant lovers: forcing. We cut dormant twigs at various times during the winter, put the twigs in containers of water, and tested how their leaf-out times responded to a variety of lab conditions.
Our results suggest that most species measure and respond to both the length of the winter and the amount of warming in the spring. For example, some woody species native to the northeastern United States, such as paper birch and wild grape, need only one or two months of winter chilling before being able to leaf out quickly when they are exposed to warm temperatures, while others, such as sugar maple and beech, need more than three months before they can start responding to spring warming. Only a few species, beech trees for instance, use the increasing day length in the spring to cue their leaf-out times. Even after fulfilling their winter chilling requirements, some species require more time in warm conditions than others to leaf out. Some species can produce a flush of new leaves after only a week in warm conditions, but other species need weeks of warm weather.
These variations in environmental cues for budburst mean a changing climate will affect each species uniquely. If we look only at winter length, sugar maple and beech will have more trouble reaching their chilling requirements for leaf emergence in the southern part of their ranges in North America as the climate continues to warm, in comparison to paper birch or wild grape, which have a lower chilling requirement. Indeed, several models of forest change show that later leaf out due to a warmer winter and earlier spring may even shorten the growing season of certain species in coming decades.
Our forcing experiments also highlighted differences important for species interactions in a changing climate. For example, many invasive shrubs, such as Japanese barberry and multiflora rose, have minimal or even no winter-chilling and day-length requirements for leafing out and will actively put out new leaves after a week of warm weather; whereas native trees of mature forests will always wait until the late spring before leafing out, even when the conditions are warm. With earlier leafing out and a longer growing season, some invasive shrubs could increase their competitive advantage due to warmer spring temperatures.
Experiments can tell us a lot about a selection of species, but it’s also important to know how large groups of species differ in their leaf-out times when growing outside. With this question in mind, in 2011 we began monitoring the leaf-out times of more than 1,000 tree and shrub species at the Arnold Arboretum in Boston. We saw many intriguing patterns, but we were unsure if what we were observing was peculiar to this one garden or was a more general phenomenon. So in early 2012 we invited botanists from around the world to join us in monitoring leaf-out times. To our pleasant surprise, almost everyone accepted our invitation. In the spring of 2012, eight botanical gardens in places as far flung as Beijing, Berlin, Munich, Ottawa, Chicago, and Washington, DC, agreed to form a team to monitor the leafing out of trees, shrubs, and vines. Our team has now recorded the leafing-out times of more than 1,600 woody plant species over several years.
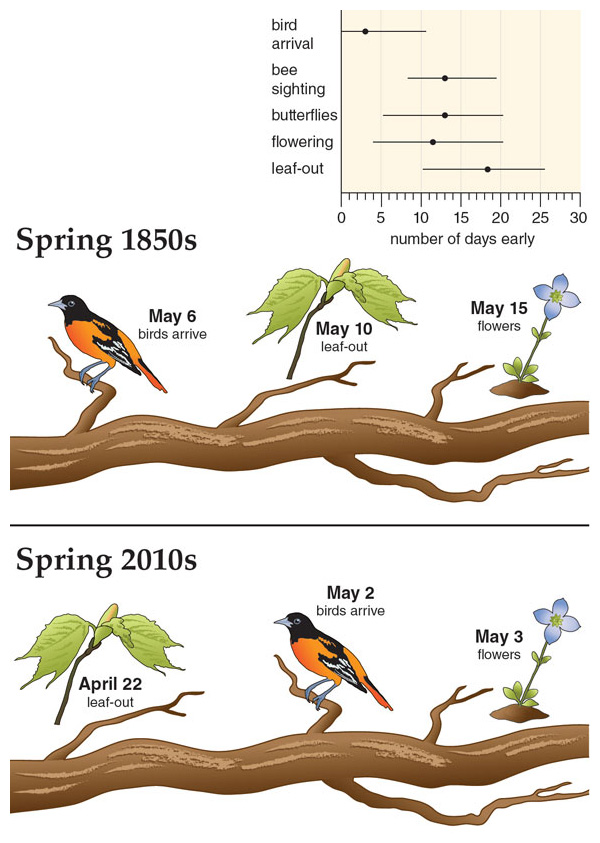
Together we have learned that species vary more in their leaf-out times than previously thought; at some of the gardens, the earliest species produced leaves two months earlier than the last species. At the Arnold Arboretum in Boston, for instance, certain species of honeysuckle, rose, and gooseberry start to leaf out in early April, but most species leaf out in late April and early to mid-May. The last plants to leaf out, including many species of rhododendron, pine, and spruce, do not do so until the end of May and early June. As far as we know, no one had ever thought to broadly monitor leaf-out times at botanical gardens before, so this huge range of leaf-out times came as a surprise. This lack of knowledge of leaf-out times contrasts with the detailed surveys that botanical gardens make of flowering times of their collections, particularly for showy species such as lilacs, forsythias, and magnolias.

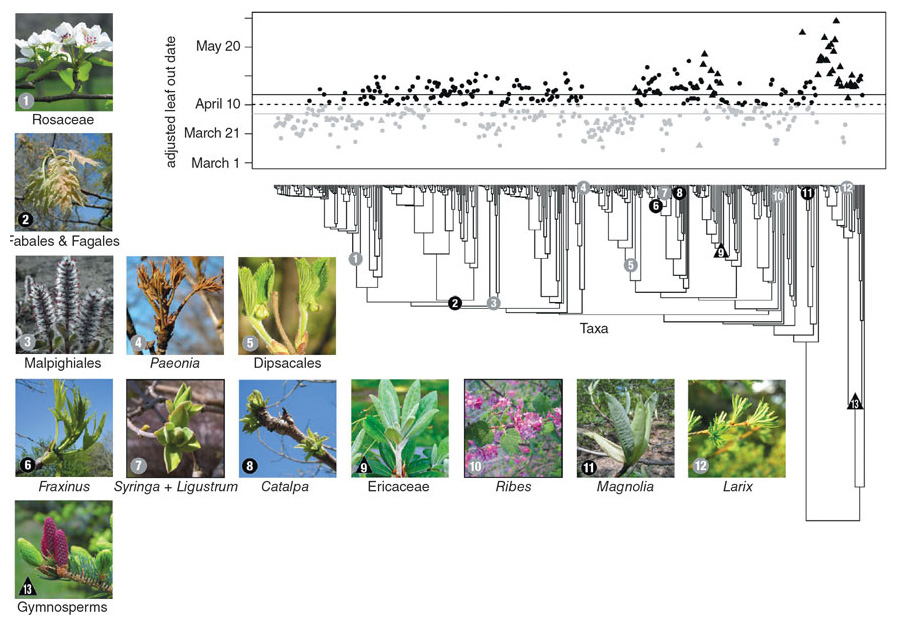
Strikingly, the order of species leafing out at any one garden is almost identical from year to year, with some species always starting the process early and other species always late. The order of species at different botanical gardens is also very consistent. For example, the species that grow at the Arnold Arboretum, the Berlin Botanical Garden, and the U.S. National Arboretum leaf out in essentially the same order at each location, despite the differences in the climates of Boston, Berlin, and Washington, DC. This unexpected consistency will simplify making short-term predictions about the effects of a changing climate. If the varying cues shuffled species’ order, any predictions would be less surefooted.
We have also uncovered intriguing patterns with ecological and evolutionary significance. First, we found that shrubs tend to leaf out before trees, which makes sense, because shrubs need to leaf out early to capture the energy of abundant sunlight before the trees above them leaf out and the canopy creates shade. Second, deciduous plants tend to leaf out before evergreen ones put on new spring leaves, presumably because deciduous species are in more urgent need of the photosynthates supplied by new leaves, whereas species with evergreen leaves can rely on the leaves from the previous year. Third, species with small xylem cells for transporting water, such as black cherries and red maples, tend to leaf out before species with large ones, such as red oaks and ashes, because small cells are less likely to be damaged by frost and air bubbles in winter than larger cells. And fourth, there is a strong evolutionary component. Certain groups of related species, such as members of the rose family, tend to leaf out early, while other groups of related species, such as oaks, tend to leaf out late.
As the climate in many areas continues to warm, the species that leaf out earliest and are most responsive to temperature will be in a better position to take advantage of an earlier spring and have a longer growing season. These species will most often be deciduous shrubs, such as honeysuckles and roses, and plants with small vessel cells, such as cherries and maples. However, this advantage will occur only if the spring gets consistently earlier; warming accompanied by an increase in unexpected spring weather could throw plants, such as red maple, for a loop. For example, earlier warm periods followed by late-season hard frosts could wreak havoc on early leaves.
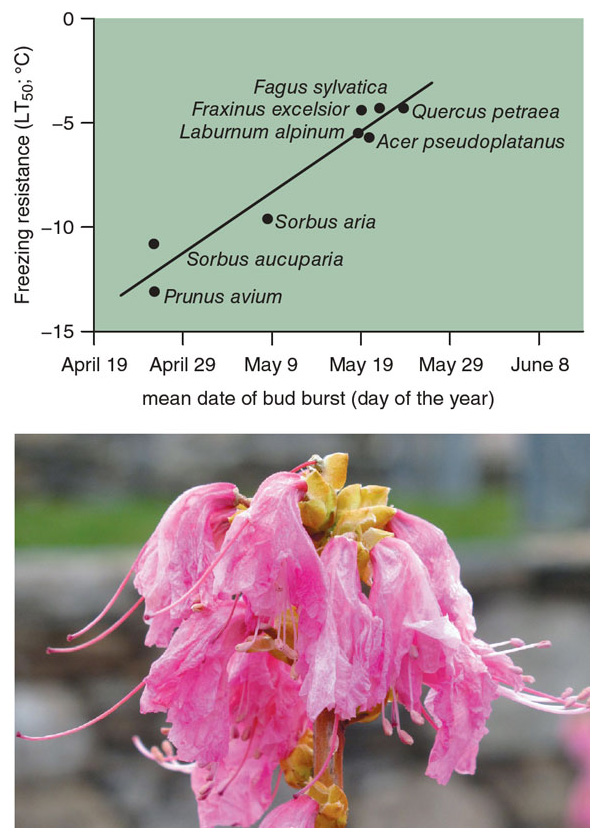
Leafing out earlier should mean that trees have a longer growing season. That change, in turn, could mean more time to photosynthesize and sequester carbon, potentially producing an environmental benefit. Species that leaf out earliest, however, are in the greatest danger of having their young leaves damaged by a late frost, which can stunt growth instead. Recent experiments from Switzerland suggest that plant species that leaf out earliest in the spring, such as members of the rose family, have a greater degree of frost tolerance in their young leaves than species that leaf out later. Therefore, the risk of frost damage depends on both annual weather variations and the overall mix of local species.
At the forest level, an exceptionally warm spring can lead to a whole forest leafing out early, but if a southward dip in the jet stream follows, creating a frost event, there can be widespread damage to vulnerable young leaves, especially sensitive trees such as certain species of oak. Late frosts are particularly devastating for fruit trees, such as apples and cherries, in which frost damage to young flowers and immature fruit can lead to a failure of the entire crop a few months later. Such an event took place across the northern United States in 2012, wiping out the cherry and apple crops in Michigan and many other regions. If a late frost damaged the flowers and young fruits of wild blueberries, there would be fewer fruits for commercial growers and also for birds such as the wood thrush to eat, with implications for adult and young bird survival. Damage from such frost events to young leaves, flowers, and fruit is predicted to become more common in coming decades due to climate change.
Spring frost events themselves are predicted to become less frequent overall in North American forests. The combination of warming spring temperatures that lead to early leaf out and flowering, and more variability in spring temperatures, however, means that the possibility of plants encountering frost when they have new leaves and flowers is increasing. One such frost occurred in 2007 across the eastern and central United States. Over an extensive area, many tree species leafed out several weeks early due to a very warm late winter and spring, including the warmest March on record, and then a late frost from April 5–9 killed young leaves, during which nighttime temperatures were consistently below –4 degrees Celsius over a large area of the eastern United States. While the trees eventually produced a second flush of leaves, the tree canopy never developed its normal abundance of leaves. Additionally, trees likely had to allocate carbohydrates stored in their roots to produce a second flush of leaves, a process that can lower their reproductive success and growth for the year and decrease reserves for future needs (such as another frost in the following year). The 2007 frost damaged the young flowers of many tree crops, such as pecans, apples, and peaches, leading to a failure of fruit production several months later.
Species that leaf out earlier in the spring have the advantage of a longer growing season. They can grow larger sooner and shade out other plants that might otherwise compete with them for sunlight, water, pollinators, and other resources. Many non-native invasive plant species, such as honeysuckle and privets, leaf out early in the spring, and this timing might be part of the reason for their success. The disadvantage of leafing out early is that such species are subject to late frost events that may kill the leaves.
Species that produce their leaves late are less likely to encounter frost events, but may also be the most vulnerable if they do encounter frost. For instance, we have found that the late-leafing tupelo tree (also called black gum, or Nyssa sylvatica) has young leaves that are heavily damaged by moderate and hard frost. The species that balance these advantages and threats best will probably be the most successful as the climate changes. The Japanese barberry, for example, which is an invasive species in North America, combines early leafing out with a high degree of frost tolerance.
Since the earliest species to leaf out in the spring also tend to be the most sensitive to temperature changes, these species are the most likely to be affected by climate change. They may advance their leaf-out times to take advantage of a longer growing season, but they also are the species most likely to encounter frost during the vulnerable stages of leafing out and flowering. Species will likely balance these challenges differently, with some succeeding and outcompeting their neighbors, and others failing to adapt to new physiological challenges.
The differences in how species respond to climate change in leaf-out and leaf senescence timing means that we can expect to see changes in interactions among species, species compositions of forests, and even the best practices for managing forests for wood production and tree orchards for fruits and nuts. To better understand species differences in leaf dynamics and how climate change will affect them, scientists are establishing extensive networks to monitor leaf-out and senescence times throughout the world, and they are finding and analyzing historical data sets such as Thoreau’s. Governments in East Asia and Europe have established particularly extensive networks to monitor leaf out and flowering of key plant species in the spring. Such data sets can provide insight into how species will respond to warming temperatures.
The basic understanding of leaf out and leaf drop that we are building allows us to make some informed predictions about how changes in temperatures will affect the growing season for particular species and groups of species. In Concord, for instance, we can say with some confidence that for each degree Celsius increase highbush blueberry’s growing season advances by about four more days than does silver maple’s. This is an important first step.
From here, we can start investigating the factors that might make some species “winners” and others “losers” in a changing climate, as well as the factors that could make some forest trees and crops resilient and others vulnerable. This area of inquiry includes research into when leaves become susceptible to frost in the spring, how the length of growing seasons changes vulnerability to drought or produces more biomass, and how interactions among species are changing.
Similar research is ongoing in desert and grassland areas, where changing rainfall patterns are more important than rising temperatures in affecting the timing of leaf out and senescence. Climate change is also affecting tropical areas, with small temperature increases already showing large effects on basic forest processes, such as the frequency of drought and the mortality of trees.
At this point we have gained considerable insight into how leafing-out times are changing over previous decades, how species differ in their leaf-out times, and what environmental factors trigger leaf out. We still need to know how such changes in leaf out will affect calculations of carbon sequestration and the relative growth and survival of different species.

Once we started looking at leaf emergence, we soon realized that we needed to look at leaves at the end of the season, too. And it turned out to be much more complicated. Leaf color changes and leaf drop are mostly occurring later in the autumn due to a warming climate. Broadly speaking, if trees hold their leaves later in the fall, the growing season is extended, which can result in trees making more wood, absorbing more carbon dioxide from the atmosphere, and providing more leafy food for animals to eat later in the season. These autumn processes, however, are far less studied and more complicated than leaf out in the spring, and we don’t have enough information to make more specific predictions.
In the spring, plants leaf out mostly in response to temperature, but autumn leaf senescence is determined not only by air temperatures but also by day length, soil moisture, frost events, wind gusts, and disease and insect attacks. As one example, a dry, warm summer leads to drought conditions that can cause leaves to drop off earlier in the autumn, but humid, warm weather can allow leaves to stay on the trees longer.
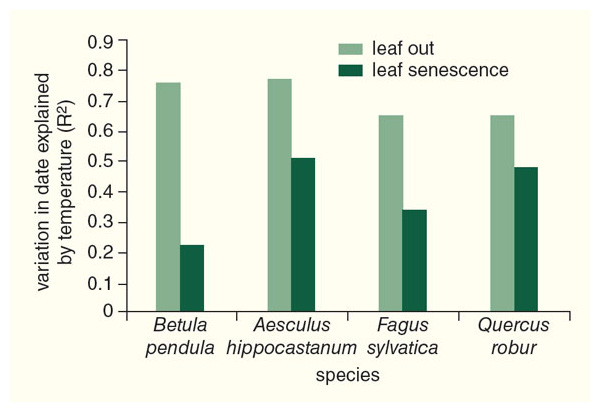
At the same six botanical gardens where we have investigated leaf-out times, we also have been monitoring leaf senescence times (meaning the process of leaf color change and subsequent fall from the tree). We have found that the patterns of leaf senescence are much more difficult to determine than what we discovered for leaf-out times. There are a few species that characteristically lose their leaves either early (August or September for species such as chokeberries and honey locusts) or late (November and December for species of privet and honeysuckle) in the autumn, but most species do not have a fixed time that they lose their leaves. The sequences of leaf senescence times is also not the same from one place to another, meaning that species may react in quite different ways to different climates and local environmental conditions.
Our colleague, Jason Fridley of Syracuse University, has come up with another intriguing result: In the northern United States, non-native shrub species hold their leaves longer in the autumn than native shrub species; thus, invasive shrubs may gain a competitive advantage in both spring and autumn from a longer growing season and greater carbon storage. Scientists at a number of institutions are actively analyzing remote sensing data from the autumn to determine environmental cues for color change and leaf drop over large areas of the northern temperate forest.
A related topic is how climate change is affecting the interaction between the maturation time of wild fruits, the migration times of birds, and seed dispersal. We know that many migratory bird species, such as the blackpoll warbler and the hermit thrush, are delaying their migration in the autumn. Yet the fruits that they feed on, such as blueberries and black cherries, are not maturing later. We are currently investigating if these bird species are increasingly relying on the late-season fruits of non-native invasive species, such as multiflora rose and Asian bittersweet, and in the process dispersing their seeds and helping these species to spread further.
It will take huge amounts of data to understand how leaf emergences and other phenomena in the spring are responding to climate change. In an effort to collect those data, participants in a number of citizen science projects are actively observing leaves all over the world. Many of these projects invite the public to make observations of plants—leaves, flowers, fruits, and other features—following standard protocols, and then to submit them to curated databases that scientists can use to inform their research. These projects include Nature’s Notebook, Project Budburst, and iNaturalist. We invite you to join us and other researchers studying the timing of leaf out and leaf drop by recording and submitting observations to these networks. Together, we can work toward a better understanding of these important processes. While these projects are unable to draw on the historical resources provided by Thoreau, they have the advantage of a much larger number of observers and locations.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.