Perceptual Pleasure and the Brain
By Irving Biederman, Edward Vessel
A novel theory explains why the brain craves information and seeks it through the senses
A novel theory explains why the brain craves information and seeks it through the senses

DOI: 10.1511/2006.59.247
As you picked up this magazine and leafed through it just now, your eye likely was drawn to certain images, words or phrases. Did you merely glance at a series of unrelated pictures as you turned the pages—or did you stop to examine one closely and read its caption? If so, what compelled you to want more? Why is it that you find the experience of looking at some illustrations or texts more engaging or rewarding than others?

Ken Redding/Corbis.
If you're beginning to suspect that this article is nothing but a marketing survey, relax! These questions are in fact matters of serious interest for neuroscientists studying cognition and perception. All of us have felt the pleasure of acquiring information—a view of a dramatic landscape, a conversation with a friend, or even a good magazine article, can all be profoundly gratifying. But why is this so? What makes these experiences so pleasurable?
We believe that the enjoyment of such experiences is deeply connected to an innate hunger for information: Human beings are designed to be "infovores." It's a craving that begins with a simple preference for certain types of stimuli, then proceeds to more sophisticated levels of perception and cognition that draw on associations the brain makes with previous experiences. When the hunger becomes even moderately starved, boredom sets in. Consider, for example, the last time you enjoyed staring at a blank wall or listening to a repetitive airport-security announcement.
In our view, infovore behavior is activated only when other motives are not engaged. When people are trying to satisfy a need for food, are avoiding harm or are otherwise involved in some goal-oriented behavior, then the infovorous instincts take a less active role. The infovore system is designed to maximize the rate at which people acquire knowledge under conditions where there may be no immediate need for the information. Of course, the knowledge obtained may have some practical value in the future. But even if there is no direct use of the new information, there is, in evolutionary terms, adaptive value to its acquisition. People generally perceive those who are knowledgeable as being more intelligent, a trait that is strongly correlated with mate selection in every human culture that's been studied.
If infovore behavior is so valuable to our species, one would expect the brain to have cellular and molecular mechanisms that encourage the acquisition of information. We believe we have identified such a system, and it is associated with a reward network that relies on the brain's natural opioids. Here we present a hypothesis for how this mechanism works. Although our model pertains to the brain's visual system, we believe that similar mechanisms may be involved in other senses as well.
There is ample evidence that the brain is wired for pleasure. Indeed, human beings have been searching for chemical substances to stimulate these neural systems for thousands of years. Among the most rewarding substances ever discovered are compounds derived from the opium poppy. Historical records of the poppy's cultivation date back to 3400 B.C. in lower Mesopotamia, where the Sumerians referred to it as hul gil, the "joy plant." However, the active ingredient of opium, morphine, was not identified until the early part of the 19th century. (Heroin, a more potent opiate, was synthesized from morphine just a few decades later.)
Barbara Aulicino
After millennia of experimentation, it's quite obvious that opiates alter the brain's activity, but it wasn't until the early 1970s that scientists understood why this is so. In 1972, Solomon Snyder and Candace Pert, at the Johns Hopkins University School of Medicine, discovered that opiates target certain molecular receptors located on the surfaces of brain cells. When opiates bind to these opioid receptors, they modulate the activity of the cells. Neurobiologists have since described at least three major subtypes of opioid receptors, identified by the Greek letters mu, delta and kappa. The mu-opioid receptors readily bind morphine, and are also activated by endogenous morphine-like substances. In 1997 these endomorphins were isolated in brain tissue by scientists at Tulane University School of Medicine.
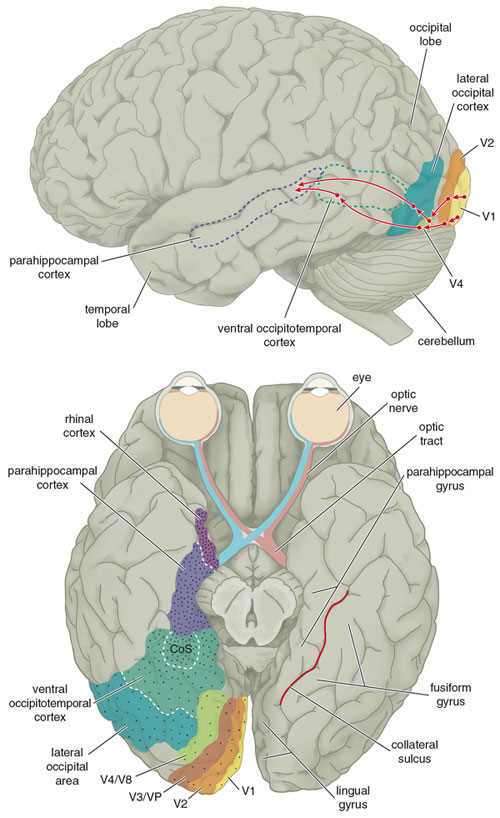
Not surprisingly, mu-opioid receptors are generally localized to parts of the central nervous system that are implicated in the modulation of pain and reward. In the early 1980s, however, Michael Lewis at the National Institute of Mental Health and his colleagues discovered these receptors in a part of the macaque monkey cerebral cortex that is involved in processing visual information. What's especially intriguing is their finding that the receptors are distributed in a gradient that gradually increases in density along the so-called ventral visual pathway, which is involved in the recognition of an object or a scene. Subsequent work has found evidence for a similar gradient in the homologous human areas. The receptors are sparsest in the early stages of this pathway, the so-called V1 to V4 areas, where an image is processed as local bits of contour, color and texture. Intermediate stages of visual processing such as the lateral occipital area and ventral occipito-temporal cortex, which integrate local information to detect surfaces, objects, faces and places, contain greater numbers of opioid receptors. The receptors are densest in the later stages of recognition, in the parahippocampal cortex and rhinal cortex, where visual information engages our memories.
Why would a mechanism that's involved in reducing pain and providing reward be associated with a system concerned with processing visual information? Furthermore, why is the greatest density of receptors found in the parahippocampal cortex, a so-called association area? We think that the mu-opioid receptors are the key to the pleasures we derive from acquiring new information, the pleasure we hope you're deriving from reading this article.
Consider the function of association areas. They are repositories for both semantic memories (such as facts and concepts) and episodic memories (such as autobiographical experiences, including the time, place and emotions associated with an event). These areas are activated when the brain tries to interpret what it's seeing or hearing. If a stimulus contains a great deal of interpretable information, it should lead to more neural activity in the association areas and hence to a greater release of endomorphins and increased stimulation of mu-opioid receptors. As more opioid receptors are stimulated, there should be a boost in the pleasant effects associated with opioids. So, for example, a visual stimulus that elicits many episodic or semantic memories should be more pleasing (or more interesting) than a stimulus that brings forth fewer mental associations.
Human beings also prefer novelty. For example, although one may thoroughly enjoy a particular conversation, the same conversation a second time around would be banal. The same can be said for any number of visual stimulations—the visual pleasure experienced on first seeing a painting, a movie or even a magnificent vista like the Grand Canyon may be unrepeatable. How does our hypothesis account for the reduction in reward when a stimulus is repeated?
Barbara Aulicino
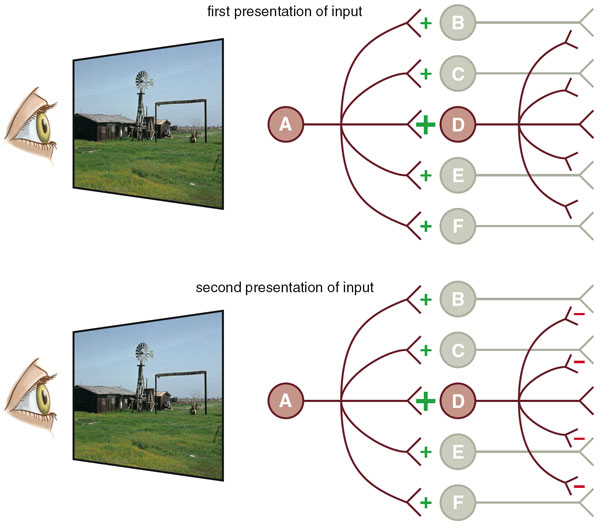
We think that a phenomenon called competitive learning may be involved. Let's assume that an initial presentation of a stimulus pattern activates a large population of neurons. A relatively small number of these cells are strongly activated, while much larger numbers are moderately or weakly engaged. One model of neural plasticity, called Hebbian learning, holds that the strength of a synaptic connection between two neurons increases with the repetition of a stimulus, so that it becomes progressively easier for a presynaptic neuron to activate a postsynaptic neuron on subsequent occasions. The greater the activity of the postsynaptic neuron, the greater the increase in the strength of the connection.
However, strongly activated neurons also inhibit those neurons that are only weakly engaged, so the repetition of a stimulus reduces the activity of these cells. With repeated presentation of a pattern, only a few neurons (those with the strongest connections) will respond, while the rest respond weakly, if at all. If most of the initial activity is generated by neurons that will be subsequently inhibited, then repetition results in a net reduction of neural activity. One advantage of competitive learning is that the inhibited neurons are now free to code for other stimulus patterns.

Images courtesy of the authors.
So our hypothesis proposes that the rate of endomorphin release in the parahippocampal cortex determines, at least partially, the human preference for experiences that are both novel (because they have yet to undergo competitive interactions) and richly interpretable (because such patterns would initially activate an abundant set of associations in brain areas that manifest dense opioid receptors).
Readers familiar with the neurochemistry of opioids, which usually act as inhibitory neurotransmitters, may wonder how opioid activity results in pleasure. It turns out that in several systems where neural connectivity has been studied, neurons with mu-opioid receptors make synaptic connections with so-called GABAergic neurons, which release the inhibitory neurotransmitter gamma-amino-butyric acid (GABA). Thus the activation of mu-opioids serves to inhibit these inhibitory neurons. It appears that much of the brain is immersed in an inhibitory "GABA bath," which probably serves to prevent seizures caused by entrained oscillations of neuronal activity. The release of endomorphins inhibits the local release of GABA and so allows local excitation to be propagated with greater intensity to the succeeding stages of information processing.
The complex response to a pleasing stimulus is mediated by a variety of cognitive, motor and hormonal pathways. We are not sure what the next stages might be, but they may ultimately increase the release of dopamine within the corpus striatum, a structure deep in the brain that is implicated in the control of movement, cognition and habit learning. The corpus striatum is directly connected to the visual areas and also to the hippocampus, amygdala and prefrontal areas of the brain, any of which could serve an intermediary role in rewarding information acquisition with a neurochemical frisson of pleasure. The release of dopamine in the corpus striatum is also believed to be involved in the cravings associated with drug addiction.
Our hypothesis would be merely another speculation about the brain if it could not be tested. Fortunately, the technology of functional magnetic resonance imaging (fMRI) allows us to observe the brain's response to a stimulus. An fMRI scanner measures the flow of blood to the brain with a precision of a few millimeters, allowing scientists to identify regions that are using significant amounts of oxygen—a measure of neural activity. With fMRI we can present a stimulus to a subject while observing neural activity in different parts of the ventral visual pathway. If our hypothesis is correct, "interesting" stimuli should elicit greater activity in the visual association areas of the brain, such as the parahippocampal cortex, but not necessarily in those areas involved with the initial processing for visual information.
Barbara Aulicino
In our experiments, we presented a series of images depicting real-world scenes to a group of subjects whose brains were not observed by fMRI, but who were asked to rate their relative preference for each picture. The images were presented several times to each subject. With each repetition, their preference for an image declined. The same images were then shown to another group of subjects lying within an fMRI scanner. These subjects viewed the images passively, without voicing their preferences. Each scene was presented for one second, and then shown again after the subject viewed an average of 15 other scenes. There were five repetitions of each scene. In order to provide consistent context for each viewing, and to pace the experiment so that an estimate of each discrete fMRI response could be extracted, we inserted "buffer" images strategically into each sequence; we also continually introduced new scenes over the course of a session, so that "first" presentations of images and subsequent presentations were scattered throughout the experiment. These tactics were also intended to remove any confounding effects of time with repetition.

Images courtesy of the authors.
As our theory predicts, scenes that were rated highly elicited the most fMRI activity in the parahippocampal cortex, especially the posterior portion. The activity in this area was not the result of a simple "feed forward" mechanism, because a region involved in the early processing of visual information, the lateral occipital area, showed its greatest activity when the subject was viewing scenes of low preference. Moreover, the activity in a portion of the ventral occipito-temporal cortex centered in the collateral sulcus and adjacent to the parahippocampal cortex, declined with each repeated presentation. This decline with repetition took place for all of the images, whether they were initially rated high or low. "Early" visual areas of the brain, such as V1 and V2, did not show a consistent decline in activity with repeated presentations.
It should not be surprising that the brain has information-acquisition mechanisms that reward us for learning about the environment. As we mentioned earlier, such mechanisms would have an evolutionary advantage. This may explain why the highly rated pictures we used in our experiments had a few things in common. Preferred pictures often contained broad views of the landscape, especially scenes that provided a hidden vantage point where an observer could view the terrain from a place of refuge. Scenes with an element of mystery, where something was happening or could happen, were also favored. Moreover, natural settings were generally preferred to man-made environments. These factors accounted for about 62 percent of the variance in average ratings. They are also consistent with anthropological explanations, which suggest that such predilections arise from a primitive need to find the best location for a camp or a village. A house on a hilltop still demands a premium in the real estate market!
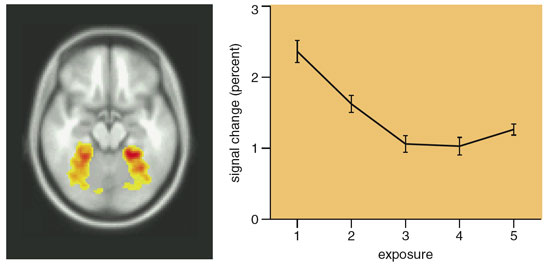
Image (left) courtesy of the authors; Barbara Aulicino
We also wish to emphasize that perceptual preferences arise from the connections the brain makes with stored information. That's because the brain's association areas have the greatest density of mu-opioid receptors. In other words, it is the interpretation of a visual pattern that leads to the feelings of pleasure. This point is nicely illustrated with the visual humor of droodles, which were popular several decades ago (Figure 8, below). By itself, a droodle is a simple pattern that elicits little in the way of a positive emotional response. Reading the droodle's caption makes it funny, because the reader activates a train of associations that reconcile what is otherwise a meaningless pattern.
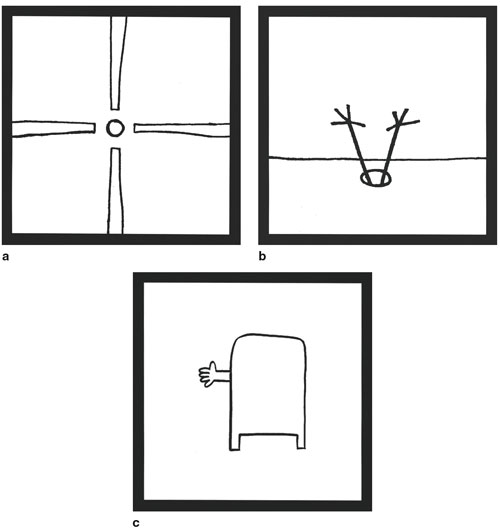
Roger Price
What about the human preference for novelty? This issue is a little more complicated than one might expect. For one thing, there appear to be some counterexamples. What psychologists call the mere exposure effect, in which the familiar is preferred to the unfamiliar, seems to contradict our hypothesis and our results. However, we believe that the key here is the subject's ability to understand the stimulus. For example, as one struggles to comprehend a new idea, there is increased pleasure with repeated exposure, which peaks at what has been called the "click" of comprehension. In our view, that click corresponds to the release of endomorphins in the association areas as the brain makes rich connections with stored information. Habituation and decline in preference may set in afterward as the associative activity becomes immediately subject to competitive learning, which reduces endomorphin release on the next exposure.
In other words, the time course of perceptual and cognitive pleasure can be described as an inverted U-shaped function. The mere exposure effect should be confined to the relatively brief, ascending initial portion of this function. Insofar as the vast range of our experiences are understood as they happen, the phenomenon of increased preference with exposure should be the exception rather than the rule.
We should add that the time course of cognitive pleasure may be somewhat protracted for children. A child may wish to hear the same story read to her over and over again (much to the chagrin of an adult reader), even to the point where sections of the story are memorized verbatim. However, when the youngster is questioned about the story—for example, why a particular character acted in a certain way—the child often reveals a lack of comprehension. It's only after a child fully understands the point of the story that she tires of hearing it again. This may be analogous to an adult's experience of mastering challenging subject matter. The payoff is in the click of comprehension, however difficult the path to that point.
It may also be the case that some childhood behavior does not engage the reward system considered here. Video games are replete with repetitive perceptual inputs that seem to be endlessly amusing to young people. We suspect that children can tolerate the repetition because they are rewarded with ever-increasing scores until the game is mastered. It would be rare for someone to seek the repetitive stimulus of a game without having access to the controller! In general, many repetitive activities expressed during childhood may serve to build motor skills or improve performance, rather than increase knowledge.
Our hypothesis also suggests that there is a preferred rate of presenting information that may correspond to the release of endomorphins. People typically experience an aversion to perceptual inputs that are presented much more slowly than the rate of comprehension. A familiar example is the impatience experienced by users of the Internet who must wait for images to be delivered over a narrow-bandwidth modem. Artifacts of popular culture—MTV videos, modern television editing and video games—are paced at the edge of the viewer's comprehension. Our work in the laboratory shows that individuals enjoy searching for target images in rapid serial presentations (about 100 milliseconds each) as long as they can maintain a reasonably high level of accuracy.
Our proposed mechanism for perceptual pleasure has focused primarily on the visual system, but we suspect that other sensory systems may have a similar arrangement. There is, for example, a mu-opioid receptor gradient in the auditory system of the macaque. Here the receptors are relatively sparse in the primary auditory cortex and denser in the secondary auditory cortex. In the early 1980s, the Stanford University psychopharmacologist Avram Goldstein reported that people who normally experienced "chills" while listening to certain stirring pieces of music did not have the same sensations when they received the drug naloxone, a mu-opioid antagonist that prevents endomorphins from binding to their receptors. Such observations suggest that, at the very least, the pleasures we associate with sounds may be mediated by mu-opioid receptors in the auditory cortex. Whether similar mechanisms are involved in touch, taste and smell remains to be seen.
In any case, our hypothesis may also extend to other forms of visual preference. For example, people enjoy viewing images in stereo; stereograms have been a popular form of amusement for over a century, even though they provide little information about a scene. Functional MRI studies show that subjects who are viewing stereo images exhibit dramatic activity in visual-cortex regions just outside the primary visual cortex. These stereo-responsive brain areas may prove to be sufficiently rich in endomorphin activity. A similar neural basis may also underlie our species' fondness for color. The lingual gyrus has been implicated in processing information about the color and texture of an image, and endomorphin activity in this region may account for this fondness (see Figure 2, bottom).
There is much that remains to be explained about the human obsession with information. Our preliminary work suggests that the quest for knowledge can never be sated—as long as mu-opioid receptors remain unbound in the human brain.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.