Narrative
By Roald Hoffmann
A good story can make up for a lack of simplicity
A good story can make up for a lack of simplicity

DOI: 10.1511/2000.29.310
What does the forthcoming political campaign have to do with tetrahedrane, a beautiful yet unstable hydrocarbon with four CH groups at the corners of a tetrahedron? Just look and listen (you'll have a hard time not doing so) to the onslaught of masterfully crafted oversimplifications thrown at you this fall by both parties. And think about why people—no, not you, of course—succumb to it. It has something to do with the reasons why we lust for the elegantly simple molecule in the shape of a Platonic solid or the beautiful (and preferably, soluble) equation. In this Marginalium I want to think about what gives us satisfaction in science when simplicity fails us, as it must, in a real world.
If one can make any generalization about the human mind, it is that it craves simple answers. The ideology of the simple reigns in science, as it does in politics. So we have the romantic dreams of theoreticians (for example, Dirac) preferring simple and/or beautiful equations. And the moment Richard Smalley, Sir Harold Kroto, Robert Curl and their coworkers intuited that the C60 peak in their laser-ablated carbon mass spectrum came from a molecule that should grace the flag of Brazil, I believed it. It could not be otherwise. And they were right.

Simplicity, symmetry and order ride a straight ray into our souls. I wonder why? Perhaps (this is far out) we have evolved a psychobiological predilection for the qualities of the world that rationalize our existence as locally contraentropic creatures that build molecules and poems. And I am a little unfair to the creative force implicit in the psychological imperative for the simple. The cult of mathematical simplicity as beauty is a reaching for essences that parallels the compact truth-telling of poetry. This is what Dalton, Dirac and Einstein aspired to. And this perspective has led to "the majesty, subtlety, and grace of science, and her deepest insights and discoveries," as Michael Fisher so aptly put it.
But what if the world is determined by us, by scientific us, to be complex, unsymmetrical and moderately chaotic? How do we find satisfaction, and I do mean psychological satisfaction, in such a world?
I think the answer is simple (I'm smiling). We construct with ease an aesthetic of the complicated, by adumbrating reasons and causes. We do so by structuring a narrative to make up for the lack of simplicity. And then we delight in the telling of the story. Nearly every seminar I go to brings evidence of this joy of story telling.
I suggest that narrative becomes the substitute for soaring simplicity in the operative aesthetic structures of chemists, and I think it's the same even for the most hard-core reductionist physicist. Continuing the story is the motive force for experimentation and the weaving of theories.
By way of example, here are three tales of chemical discovery:
Insects are the greatest chemists. In 1966 R. S. Berger identified the main sex pheromone of the cabbage looper moth, Trichoplusia ni (Noctuidae) as (Z)-7-dodecenyl acetate. Those were the halcyon days of early pheromone chemistry; everyone was happy with one molecule (as they were with one gene for each trait). Thirteen years later L. B. Bjostad et al. identified a second component, important especially in close-range courtship behavior—simple dodecyl acetate. Bjostad, C. E. Linn, W. L. Roelofs and their coworkers, at the New York State Agricultural Experiment Station in Geneva, New York, then began to think through the biosynthetic relations between these two components and other molecules observed in the pheromone gland. Obviously, enzymes that shorten molecules, reduce or acylate them, remove hydrogens—all the wondrous machinery of the living—are at work. Figure 2 is a complex graph from one of their papers, showing the biochemical relations between the various kinds of fatty acids present. A blend of six components, suggested by their analysis, elicited complete courting flights against a stiff breeze in a wind tunnel. Clearly one needs six for sex. And would a human master perfumer be surprised?
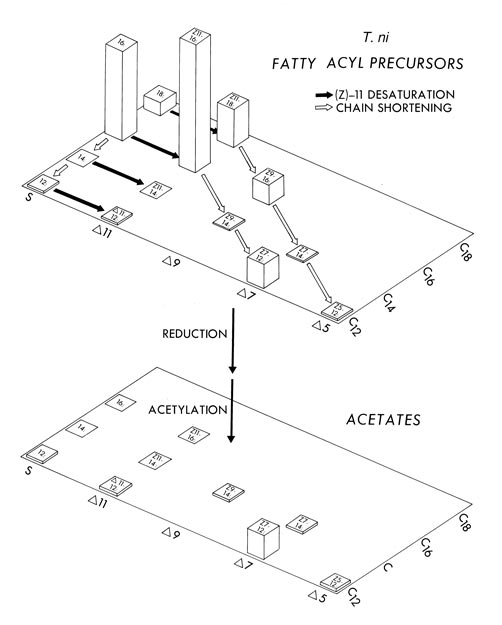
The story is told with sufficient verve in the Bjostad, Linn, Du and Roelofs paper that even I, an outsider to the field, am pulled in by it. More than just an analysis of pheromone glands, the biochemical relations are clever. I am intrigued by their tale and begin to think of its sequel—how do the females evolve that blend? How do the males evolve the receptors to it? Thomas C. Baker and his coworkers at Iowa State University have actually located separate compartments for the six components (and one antagonist) near where the male antenna input is first processed.
Blood Red: Hemoglobin is the stumbling block to the simpleminded; there is a story in every turn in this best known of proteins. I will pick one of the oldest, that of the cooperativity of oxygen uptake by this molecule. Hemoglobin has four subunits, two consisting of 146 amino acids, two of 141, each cradling a porphyrin where an oxygen molecule binds to an iron atom. Oxygenation of one subunit makes the subsequent oxygenation of another easier. An important early phenomenological theory accounted for the kinetics. But how does it happen on the molecular level, over a distance of 25–30 angstroms between iron atoms?
Max Perutz, whose perseverance and talent first gave us the structure of hemoglobin, also built a bold theory of the cooperativity. It begins with an iron atom on one subunit moving into the heme plane upon oxygenation, pulling a proximal histidine with it. Movement of the histidine shifts a helix, it is suggested; eventually a geometry change at the subunit interface ensues, where a salt bridge between subunits is broken. A net conformational change in the protein occurs, influencing the binding of the next oxygen. Is this a Rube Goldberg (Heath Robinson in England) machine? Rube Goldberg studied some chemistry at Berkeley—maybe he learned something about reaction mechanisms. It is a mechanism, a story well told, remarkably convincing. And permitting elaboration, as work by Martin Karplus and his collaborators shows.
The Road to Cariporide: Once upon a time (1986), in a pharmaceutical company (Hoechst, now Aventis), the chemists Hans-Jochen Lang and Heinrich Englert became interested in the sodium (Na+)-hydrogen (H+) exchange (therefore called NHE) system, a fine biochemical machine for moving about protons and sodium ions, and thereby regulating cellular acidity. NHE had been first described in 1976 by Swiss physiologist Heini Murer as an ion-transport system in the proximal tubule of the kidney. There were many speculations about the role of this device, present in virtually every type of mammalian cell. For instance, might NHE affect the pathophysiology of brain edema caused by stroke?
Pharmacologists and chemists started looking for NHE inhibitors. As is often the case in drug development, the problem is not so much the chemical compounds to test, for chemists have certainly learned the lesson of Genesis, that we have been put on this earth to create. No, the problem is so often the assay. In the case at hand, a promising one, using renal membrane vesicles, proved insensitive.
At the same time Hoechst pharmacologist Wolfgang Scholz was working on a completely different system, the use of ion transport in red blood cells as an assay for the identification of diuretics. One day he was asked by a cardiovascular-research group to test the red blood cells of rabbits on a high-cholesterol diet for possible changes in their ion-transport mechanisms. Remarkably, whereas NHE activity is quite low in red blood cells under normal conditions, there was an approximately tenfold increase caused by the special cholesterol-rich diet.
Whatever the reason for the original experiment (rabbits emulating American junk-food consumers?), the Hoechst scientists saw an opportunity—these red blood cells provided an exquisitely sensitive NHE assay, 1,000 times as sensitive as the kidney membrane vesicles.
There was now momentum for synthetic chemists to ply their art. New classes of compounds were tried. One pharmacologist was reading a paper in the Russian literature, on a totally different subject, when he came across the statement that a sodium ion was roughly of the same size as a guanidinium group. Now that turns out to be somewhat farfetched, but no matter, it gave impetus to the synthesis (and testing with the new assay) of a variety of guanidine derivatives. Some of these compounds, the benzoyl guanidines, turned out to be potent and specific enough to test them for reduction of brain edema. The results were quite disappointing.
Thus, in late 1988, Lang, Englert and Scholz had in hand a new class of ion-transport inhibitors. The only problem was that there were no known clinical indications for them! It was then decided to test one of the best compounds in a broader range of pharmacological models. One of them was the isolated working rat heart in the lab of the pharmacologist Wolfgang Linz. When a benzoyl guanidine code-named HOE 694 was tested in this model, Linz was amazed to find it was about the most protective compound in cases of cardiac ischemia/reperfusion (blood vessel constriction and blood resupply) that he had ever seen.
At this point molecular biology kicks in. In 1989 the group of Jacques Pouyssegure in Nice cloned the NHE gene. In the following years, several subtypes of NHE were identified. A collaboration between the Hoechst team and Pouyssegure's group soon determined that NHE subtype 1 is not only ubiquitous but also the predominant subtype in the heart and blood cells. It was found that HOE 694 and most of the related benzoyl guanidine compounds were quite selective inhibitors of NHE subtype 1. The predominant subtype in the proximal tubule of the kidney was NHE-3. Compounds like HOE 694 were about 1,000 times more effective on NHE-1 than on NHE-3. So, finally, it was understood why the red-blood-cell assay had worked and the renal membranes had not!
All the laws that characterize the infinity of failures facing human beings apply to pharmaceutical research as well. Within weeks it was revealed that HOE 694 formed a metabolite that concentrated in the rat kidneys and precipitated in the tubular system, where it caused obstruction and inflammation. A strategy to construct compounds metabolized in a different way led to a new compound, HOE 642, synthesized by chemist Andreas Weichert. Now, HOE 642 (generic cariporide) has reached a late stage of clinical development and will it is hoped come to the market in three years. I have recounted three tales of discovery. They start simple, yet in each, the ornery complexity of the real is parlayed by the protaganist chemists into a delightful, deeper story.
Everywhere one looks in science, there are stories. I could have recounted the grand ones, of the inflationary universe, of evolution, of continental drift, of Fermat's theorem. I could have told about smaller ones, no less thrilling—the quest for octanitrocubane, the European duplication of Chinese porcelain or the discovery of sulfa drugs. I could have recounted Primo Levi's stories, of a" solitary chemistry, unarmed and on foot, at the measure of man."
All of these stories have the hallmarks that literary theorists have seen in narratives, small and grand:
—Temporality: a peaceful beginning, a disequilibrated, tense middle and a resolution that often sets the world upside down.
—Causation: essential in science, the most deterministic of narrative genres. Every thing must have reason, or why would you tell it to your peers?
—Human interest: Reflect—how much more interesting are lectures than articles? Our microsociety's ossified strictures on what should go into a paradigmatic article are relaxed in seminars. One tells a story, and the audience drinks it up, for it sees the why and wherefore. And the speaker naturally tells a heroic tale of blind alleys, serendipity, obstacles overcome and all-conquering logic. Who needs a samurai epic when I can hear Sam Danishefsky or K. C. Nicolau struggling with the synthesis of calicheamycin?
At times, when I've spoken of narrative as a motive force to scientists, I've encountered a certain queasiness. Could it be that if we admit we tell a story of our research, that we get uncomfortably close to "just so" stories, inventions, fiction? Or, God forbid, that we should render support to relativists, that nefarious social-construction-of-science gang?
Relax. What we study is real. Yet we live in a mansion furnished with real things and an infinity of mirrors. Modern science is a successful social invention for acquiring not truth, but reliable knowledge (to borrow a phrase from John M. Ziman). An essential part of the structure of science is a built-in alternation of flights of wild theoretical and narrative fancy with experimental probing of some underlying reality. The fancy is not unfettered. In the pursuit of the art, craft, science and business of chemistry, there are numerous checks with reality. To be sure, each is individually deconstructible, but their totality shapes a pretty reliable network of knowledge.
But this in no way precludes tall, fancy and mythical stories that fit into absolutely every category of folktale you have, for it is human beings that seek reliable knowledge. So we clothe the oral and written reports of our curious exploration with the fabric of narrative. Narrative is absolutely indestructible; it looms just under the surface in the driest chemical article. And I am so happy that I am privy to the codes, so that I may see the myth (and the approach to reliable knowledge) underneath.
John Polanyi has recently described the close relationship between science and story-telling:
Scientia is knowledge. It is only in the popular mind that it is equated with facts. This is, of course, flattering, since facts are incontrovertible. But it is also demeaning, since facts are meaningless. They contain no narrative.
Science, by contrast, is story-telling. That is evident in the way we use our primary scientific instrument, the eye. The eye searches for shapes. It searches for a beginning, a middle, and an end.
The power of stories may indeed exceed that of facts. As Walter Benjamin has written:
The value of information does not survive the moment when it was new. It lives only at that moment; it has to surrender to it completely and explain itself to it without losing any time. A story is different. It does not expend itself. It preserves and concentrates its strength and is capable of releasing it even after a long time.
In telling the story of scientific discovery, we form a praiseworthy bond with literature and myth, all the other ways that human beings have of telling stories. Yes, there are times when the story has to be told simply, the fire engine sent the shortest route to the fire. But a world without stories is fundamentally inhuman. It is a world where nothing is imagined. Could a chemist be creative in such a world?
© Roald Hoffmann
I am grateful to Wolfgang Scholz and Hans-Jochen Lang for telling me the story of cariporide, and Wendell Roelofs for that of the T. ni pheromone.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.