Making Biofuel from Microalgae
By Philip T. Pienkos, Lieve Laurens, Andy Aden
So much potential coexists with so many scientific, environmental and economic challenges
So much potential coexists with so many scientific, environmental and economic challenges

DOI: 10.1511/2011.93.474
The drive to develop and expand alternatives to fossil fuel engages scientists and entrepreneurs around the world at levels never before witnessed.
Increasingly, consumers are being urged to imagine a future when their vehicles and commercial machinery are powered not just by gasoline or traditional diesel but also by liquid biofuels; electricity generated by wind and solar; and, perhaps, even hydrogen. Ethanol, a gasoline replacement usually made with corn in the United States, already replaces nearly 10 percent of U.S. gasoline. But researchers have made a strong case that multiple types of biomass feedstock are needed to create adequate supplies of biofuel.
The report “Biomass Feedstock For a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply,” published in 2005 by U.S. Department of Energy and Department of Agriculture researchers, has just been revised. Widely known as the “billion-ton study,” the update indicates that as much as 1.6 billion tons of terrestrial biomass from agricultural wastes, forestry waste, municipal solid wastes and energy crops such as miscanthus and switchgrass could be harvested sustainably in the United States annually for biofuels, bioenergy and bio-based products. Considering the theoretical fermentation yields on biomass sugars and the energy content of ethanol, this projection also establishes the theoretical maximum production of bio-based gasoline equivalents at close to 96 billion gallons. Since the United States uses approximately 140 billion gallons of gasoline, 40 billion gallons of road diesel and 20 billion gallons of jet fuel (all derived from crude oil) per year, it is clear that biofuels based on terrestrial feedstocks can never meet that demand. At the National Renewable Energy Laboratory (NREL), where we conduct our research, we concluded the same thing when the original billion-ton study was released. That prompted us to rebuild the Aquatic Species Program, previously funded from 1978 to 1996 by the U.S. Department of Energy, to evaluate the potential of algae-based biofuels.
Oxford Scientific/Photolibrary
We are confident that lipids derived from algae hold great promise as a supplemental biofuel feedstock. Algae have many inherent advantages in this context, with the high-lipid content found in some species being a fundamental edge. Another advantage is algae’s high per-acre productivity. Also, since microalgae are not a common food source, algal cultivation for fuel is unlikely to interfere with food production at the levels that cultivation of other feedstocks, such as corn, might. Because algae grow in many different environments, it could be produced on acreage that is not agriculturally productive. Algae farming could also make use of multiple types of water: fresh, brackish, saline and wastewater. It is widely believed—though research is needed to confirm this—that the use of algal-based fuel would result in a tiny fraction of the net greenhouse gases that can be traced to fossil-fuel use today. And scaling up algae farming could lead to yields of other commercially viable products besides fuel.
All this promise is conditional, of course. Many scientific, environmental and economic hurdles stand between today and a time when the world’s population is reaping benefits from algae-produced fuel. Highly productivity algal strains must be identified. New and reliable algae-farming methods must be developed. A means must be found to farm algae with the limited amount of water that’s available for the job. Hyper-efficient systems for extracting lipids and any other commercial products grown in algae must be invented too. If all that can be accomplished, there is still one potential deal breaker. All of this must be done at a cost that makes algae-derived biofuel competitive with petroleum-based fuels. Research at NREL is attempting to address some of these challenges.
First, it’s important to get more specific about what type of algae has landed in the alternative-fuel spotlight. Macroalgae, the seaweeds, grow in open waters, both fresh and marine. These aquatic plants are made up mainly of carbohydrates and have been harvested for centuries as food, including the nori used to wrap sushi, and thickening agents such as agar. The Aquatic Species Program explored the potential of macroalgae as fuel but dropped that project due to the significant challenges related to harvesting costs and fuel conversion. Microalgae, on the other hand, are unicellular photosynthetic microorganisms. They are ubiquitous in nature, found in freshwater, seawater, hypersaline lakes and even in deserts and arctic ecosystems. They can be further subdivided into two main categories: eukaryotic algae, possessing defined organelles such as nuclei, chloroplasts, mitochondria and so on, and prokaryotic algae (cyanobacteria or blue-green algae), possessing the simpler cellular structure of bacteria. Although the relatedness of cyanobacteria to nonphotosynthetic bacteria allows for exploitation of genetic-engineering technologies and makes them an attractive starting point for biofuels research, they lack one very important thing that eukaryotic microalgae can possess in abundance—neutral lipids, which are rich in triacylglycerols (TAGs).
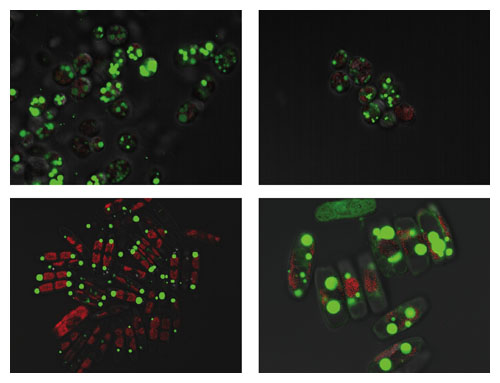
Photomicrographs courtesy of Lee Elliott.
Of the eukaryotic microalgae, green algae are the taxonomic group most often referred to as oleaginous, or oil-rich, microalgae. They are ubiquitous in a variety of habitats and grow faster than species from other taxa, and as much as 60 percent of their cell dry weight can be oils. However, the composition of the oils is highly dependent on the species and the conditions in which the algae grow. Oils that are rich in neutral lipids are desirable in a biofuel context because of their potential high fuel yield. Because TAGs are made up of three molecules of fatty acids that are esterified—or altered—to one molecule of glycerol, close to 100 percent of their weight can be converted into fuels. With polar lipids, on the other hand, only one or two fatty-acid molecules are esterified to glycerol and the remaining components (e.g. sugars or phosphate groups) cannot be converted to fuel feedstock. As a result, these types of lipids generate lower fuel yields.
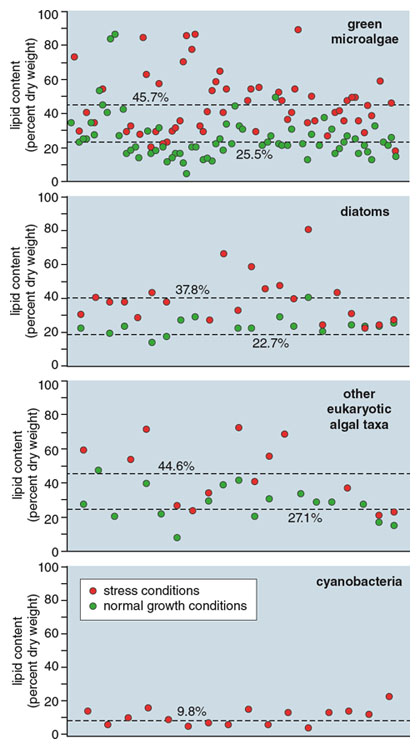
Illustration by Barbara Aulicino. Data from the article “Microalgal Triacylycerols as Feedstocks for Biofuel Production: Perspectives and Advances,” by Qiang Hu et al. 2008. The Plant Journal.
Fatty acids, the building blocks for lipids, are synthesized by enzymes in the chloroplast, of which acetyl-CoA carboxylase (ACCase) is key in regulating the synthesis rates. When cells are actively growing, their metabolic focus is on photosynthesis and the production of biomass. The fatty acids produced are mostly found in polar-membrane lipids, such as phospho- and glycolipids, which are invaluable to photosynthesis. Unfortunately only about 30 to 50 percent of polar lipids can be converted into fuel molecules. But when the cells experience metabolic stress, such as a lack of essential nutrients, including nitrogen, cell metabolism is redirected to reduce the growth rate and favor the production of carbon-storage compounds, mainly carbohydrates and TAGs. Little is known about the regulation of TAG formation at the molecular and cellular level, but greater understanding could lead to the engineering of algae with higher ratios of neutral lipids.
Organic solvents can extract oils from actively growing cells. But oils extracted from stressed cells yield more fuel. In Chlorella vulgaris, a strain that our laboratory has studied extensively, the extracted oil content amounts to about 30 to 50 percent of the biomass under both active-growth and nutrient-limited conditions. However, the fatty-acid content, reflecting the potential fuel yield, can vary from 10 to 50 percent of the biomass over the growth cycle. This illustrates the big discrepancies often seen between the extracted algal oils and the actual fuel-yield potential.
Unlike typical terrestrial oil-producing plants, in which specialized cells yield oils, every algal cell can produce oils. Algal oils, just like oils produced by soy, canola, palm and the less-known jatropha plants, can be made good biodiesel feedstocks through transesterification. In that process, a catalyst creates a biodiesel fuel (consisting of fatty acid methyl esters) by hydrolyzing and methylating fatty acids in the oils. Refining the mixture is typically the next step and involves removing the non–fatty-acid components—such as glycerol, polar lipids and residual pigments—from the fuel. Typical refinery processes such as hydrotreating, cracking and isomerization of the algal oils can also be used to produce renewable gasoline, diesel or jet fuels. These so-called drop-in fuels are much more like traditional petroleum-based fuels and can be blended, like for like, in existing fueling infrastructure.
Once algal oils have been extracted with organic solvents or removed in some other way, the remaining biomass will be made up of approximately equal amounts of carbohydrates and proteins. We expect that this residual material can be used as a feedstock for so-called coproducts to help the overall economics of algae farming. Carbohydrates can be used to produce methane by anaerobic digestion or ethanol by fermentation. Proteins can be used for animal feed or even human food. Other higher-value algal products such as omega-3 fatty acids and antioxidants are already available commercially, but the potential market for biofuels dwarfs market for these nutraceuticals. The search for high-value coproducts with large market size remains an elusive goal for an integrated biorefinery based on algal biomass.
Research published this year by Mark Wigmosta and coworkers at the U.S. Department of Energy’s Pacific Northwest National Laboratory evaluated the amount of land available for cultivation of microalgae and the availability of needed inputs such as water, carbon dioxide and inorganic nutrients—basic requirements for algal growth. This report used fairly conservative assumptions for algal growth rates and lipid content, based on current technologies, and arrived at a value of 57 billion gallons of lipid-based algal biofuels per year. Thus algae represent a feedstock source that could be comparable in size to all the terrestrial biomass that could be harvested for biofuel production combined.
In order to produce that much algal biomass, it will be necessary to develop a new type of agriculture, comparable in scale to the amount of U.S. farmland devoted to growing corn, but focused on a microscopic crop. That will require novel methods for cultivation, harvesting and processing. As anyone with a poorly maintained swimming pool can attest, algae can grow without much prompting. But this agriculture will require crops that grow at maximum rates and achieve the highest possible concentration of cells per liter of cultivation medium. Successful algae crops must be able to thrive in the presence of pests, predators and pathogens. These include “weed” algal strains that are more robust than production strains but that are of no use in biofuel production, grazers such as rotifers, and infectious agents such as bacteria, fungi and viruses. All this will require a carefully engineered cultivation process.
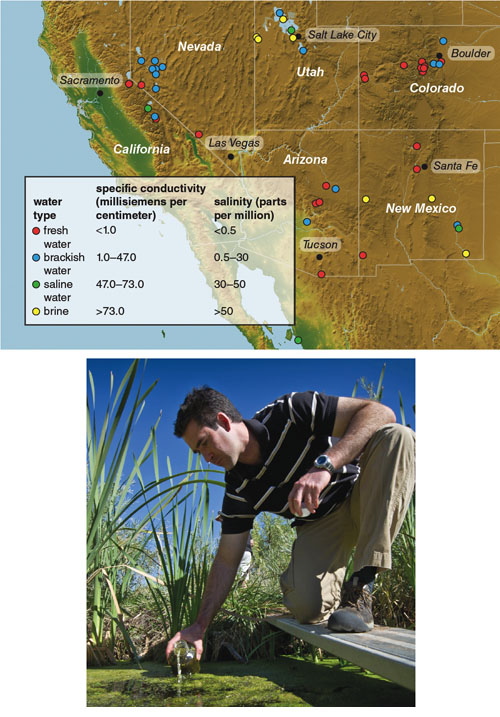
Illustration by Barbara Aulicino, adapted from a texture map by Planetary Visions Ltd.
Photograph by Dennis Schroeder, courtesy of the National Renewable Energy Laboratory.
Much of our research focuses on the growth rate and lipid content of algae. At this stage, that includes bioprospecting—the search for natural strains with high growth rate and high lipid content, as well as robustness. We look for species that are resistant to pests, predators and pathogens and have the ability to thrive under environmental conditions—sunlight, temperature and water chemistry—expected at a cultivation site. In this search, we use technologies developed for the biotechnology and pharmaceutical industries, such as robotics, liquid handling devices and fluorescence-activated cell sorting, to speed up the isolation of single algal cells from environmental samples and to test them for growth rate and lipid content. Learning from colleagues affiliated with the Culture Collection of Algae at the University of Texas, we developed methods to preserve culture samples cryogenically and to revive them at will, eliminating the laborious and sometimes counterproductive practice of maintaining algal cultures on agar plates and slants, which requires regular transfers to fresh media.
Our work in bioprospecting and our involvement with the Sustainable Algal Biofuels Consortium, led by Arizona State University, have made it clear to us that there is a widespread need to accelerate the quantification of lipids and tailor the analysis to rapidly screen several hundreds or thousands of individual strains, which is not feasible with traditional gravimetric or chromatographic separation processes. At NREL, we have developed rapid, infrared-spectroscopic, high-throughput methods for the estimation of algal lipid content based on multivariate calibration models. We have demonstrated the applicability of such methods to the quantification of exogenous lipids in algae. And we have applied improved methods to hundreds of algal biomass samples from more than 80 species, with lipid content varying from 10 to more than 60 percent over the growth of the culture. This method can distinguish between neutral and polar lipids, which is difficult using standard techniques, and could facilitate high-throughput screening to detect promising algal strains in metabolic engineering or bioprospecting projects.
In addition to C. vulgaris, mentioned above, NREL researchers are also evaluating other eukaryotic algae, including Chlamydomonas reinhardtii and species of Scenedesmus and Nannochloropsis. All three are well-researched laboratory strains. C. reinhardtii does not make much oil but is probably the most common eukaryotic algal lab strain. Its genome has been fully sequenced and many tools are available for genetic manipulation. Many of the Scenedesmus and Nannochloropsis cultivars have been exploited for oil production because both strains grow well in large-scale open ponds and photobioreactors, and both can make a significant amount of oil. Although algal researchers around the world are nowhere near exhausting the algal diversity that nature provides, many of us are also working on projects to improve on natural algal strains by classical genetics (mutation, selections, screening and breeding) as well as by genetic engineering. We are working on a number of “omics,” or system-biology projects, using genomics, transcriptomics and proteomics to understand the molecular details of high-lipid productivity and to use that information to inform experiments in genetic engineering. Goals include turning up or down gene-expression levels so that higher lipid levels can be achieved under conditions that allow rapid growth and to generate strains that are more amenable to harvesting. One example of our genetic-engineering research involves the cyanobacterium Synechocystis PCC 6803. Although untransformed cyanobacteria do not produce TAGs, we are engineering a species that can do so by redirecting carbon metabolism from carbohydrate production to fatty-acid production and by inserting missing genes for TAG production.
Genetic engineering can be a controversial topic because of fears that large-scale cultivation could result in the release of engineered strains whose recombinant genes could infiltrate other species. Regardless of whether engineered strains are ever allowed outside laboratories (where strict guidelines are in place to prevent accidental release), genetic-engineering research is critical for understanding the potential of a single algal strain to have all the properties needed for large-scale oil production. This research must be done in parallel with studies using natural strains or strains derived from classical genetics and breeding to understand the risks and to put safeguards in place for large- scale cultivation.
Two basic cultivation systems have been developed for photosynthetic microalgae agriculture: open ponds and closed photobioreactors. The ponds can be simple and shallow, 20 to 30 centimeters deep, with little or no mixing. Or they can be oval raceways with dividers in the middle and paddlewheels to keep the water moving. Ponds represent the cheapest possible system (especially if they consist of simple trenches in the ground with no liners), but they provide limited protection against pests, predators and pathogens that can drop from the air. Ponds also tend to have a relatively low surface-to-volume ratio. In dense cultures, too many algal cells can get stuck in the shade, especially without adequate mixing.

Photograph courtesy of Qiang Hu of Arizona State University.
Closed photobioreactors must be constructed of clear materials such as glass or plastic and can come in a variety of configurations, such as flat panels, tubes or simple plastic bags that either hang from a support or lie on the ground. Photobioreactors can have higher surface-to-volume ratios, so they reduce self-shading. The closed design can also help keep out unwanted organisms and reduce the amount of evaporation (thus reducing the amount of water needed for continuous cultivation). But they can have problems with CO2 transfer and with heat and O2 buildup. And they are also much more expensive than ponds. Even though the cost of production of algal biomass for high-value products such as nutraceuticals and food supplements is not as critical as it will be for fuel production, most commercial algae enterprises use open ponds. They control the quality of the crop by choosing conditions that encourage the growth of the production strain rather than the drop-ins.
Our evaluation of production economics indicates that open ponds will be much more cost effective than photobioreactors, but our laboratory hasn’t yet advocated for one technology over the other. Both systems have advantages and disadvantages, and both, at this stage, are too expensive to produce biofuels that are cost competitive with petroleum-based fuels. We will continue to monitor developments that will either reduce production costs or help defray those costs, with other improvements or by identification of value-added coproducts.
Cultivation is only one of the challenges to scaling up algal-feedstock production. Harvesting the cells and removing water to facilitate TAG extraction pose commercialization hurdles. Even under ideal growth conditions, it is difficult to get more than 1 to 2 grams of biomass per liter of culture. Aerobic fermentation processes with industrial bacterial strains such as E. coli can reach 100 grams per liter. The low algal-cell densities require as much as a hundredfold concentration of algal cells before the extraction process can be performed efficiently. Centrifugation can easily achieve this sort of concentration, but it is considered too capital- and energy-intensive for use in fuel production. Other methods such as flocculation and dissolved-air flotation have been adapted from the wastewater-treatment industry and are much cheaper. Flocculation uses inorganic ions or organic polymers (or in some cases takes advantage of the intrinsic properties of the algal cell wall) to get cells to clump together. The clumps can be collected by gravity settling or can be brought to the surface by bubbling with air or other gases—dissolved-air flotation. Both of these methods are less expensive than centrifugation but may require modifications for each algal strain and each set of growth conditions, and achieve just a fraction of the cell concentration that centrifugation produces.
Once algal cells have been adequately concentrated, it is still not easy to extract lipids. Vegetable oils can be removed by pressing seeds, but algal cells are too small and tough for efficient pressing. Hexane solvent extraction is useful with soybeans and other oil seeds, but it can be difficult to get the hexane to penetrate the algal cell walls. Often additional steps, such as sonication or mechanical homogenization, are needed to disrupt an algal cell wall and make the lipids more accessible. These steps add to the overall cost and energy requirements, so the search goes on for the process that works at scale with wet algae.
Although lipids are the key algal component for biofuel production, algal carbohydrates and proteins could be feedstock for other energy products. Methane can be produced with anaerobic digestion, and other biofuels can be made using fermentative and catalytic processes. Given this potential, NREL is also developing a strategy for accurate and detailed carbohydrate and protein quantification in algal biomass.
The technical hurdles described above have all been overcome to some degree (in the laboratory or in pilot facilities), but the biggest challenge to the commercial viability of algae-based biofuels is to carry out steps at low enough cost to produce a competitively priced biofuel. Analyses over the past 20 to 30 years predicting cost have ranged from what could be characterized as wildly optimistic (less than $1 per gallon) to more conservative but hardly encouraging (more than $40 per gallon). Until true production costs are understood, cost reduction from research and development gains are difficult to quantify. Therefore, NREL is attempting to establish baseline costs for producing algal biofuels using technology readily available today.
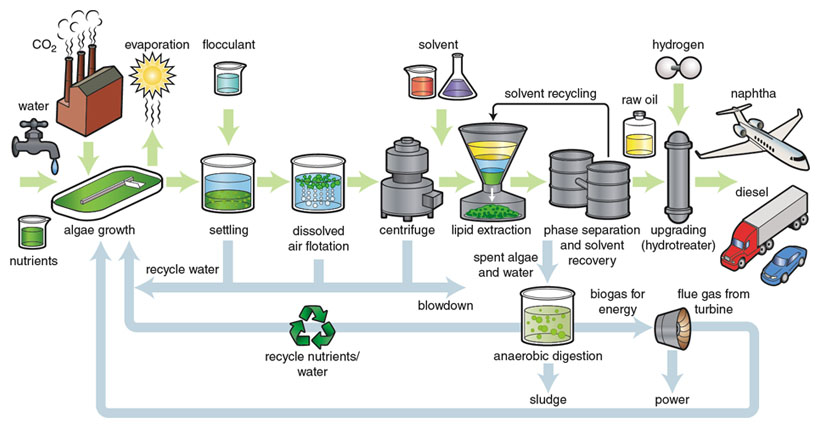
Illustration by Barbara Aulicino.
To do this, we apply experience and methodologies used previously for technoeconomic analysis of lignocellulosic, or woody, feedstocks for biofuels production. Technoeconomic analysis combines detailed conceptual process design with economic analysis to tie performance to cost. To do this, we have modeled baseline algae processes for both open-pond and closed photobioreactor systems. The models include growth; harvesting; concentration; lipid extraction and recovery; and conversion to fuel. The baseline models currently assume that spent algal biomass goes through anaerobic digestion to recover some of the energy value as methane. However, the models will allow alternative coproducts to be explored. The material- and energy-flow rates calculated from these models can be used to determine the size of equipment needed and to develop capital and operating costs for the algal biorefinery. In the end, the algal oil is assumed to be converted to diesel or jet blend stock.
In our recently published baseline analysis, fuel product at a 10-million-gallon-per-year facility could be produced for between $10 and $20 per gallon. Although many parameters affect the economics, we have identified two key cost drivers: lipid content and growth rate. With improvements to these and other parameters, as well as several coproduct scenarios, the potential for cost reduction is significant. Further enhancement of these models will come from using more experimental data from pilot operations underway across the country. Assumptions regarding the recycling of nutrients and water to reduce cost and improve sustainability will be tested for validity.
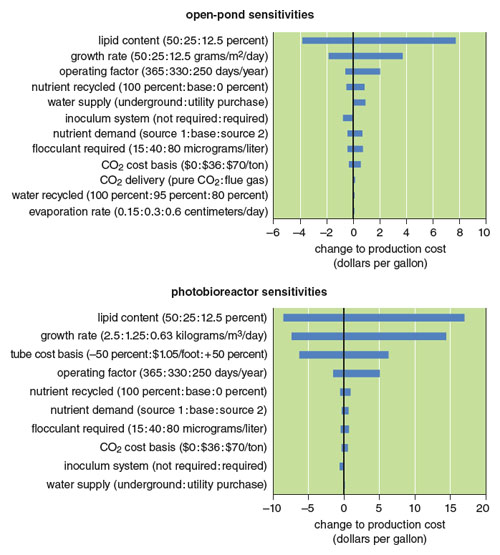
Illustration by Barbara Aulicino.
The composition of the biomass produced by a given process hugely influences the economics. The ratio and composition of proteins and carbohydrates in a given algae crop will determine the fate of the residual biomass once lipids are extracted. That can play a significant role in the overall process, perhaps even driving the development of alternative uses of residual algal biomass. For example, biomass with high fermentable sugar content could be converted into fuel ethanol, a product with more commercial value than methane.
One problem that also must be tackled is the large range in reported algal lipid content in the literature. The use of a wide variety of extraction methods and solvent types is part of the problem. The lack of a standard lipid-quantification procedure, differences in compatibility of the polarity of the solvents, differences in the polarity of the lipid molecules, and the accessibility of the lipids to solvent penetration all play a role. Inevitably, the extractable oil fraction will contain nonfuel components (such as chlorophyll and other pigments, proteins and hydrophobic carbohydrates). Thus it is necessary to assess the fuel fraction—the fatty-acid content of extracted lipids—within these oils. This is vital for accurately capturing productivity improvements. We must be certain that an observed increase in extracted lipids is not an artifact of the measurement process.
In this context, the algal-biofuels research community is moving away from extraction-based lipid quantification and toward a whole-biomass transesterification process, which gives an accurate yield of the potential fuel fraction. It does that by measuring only the fatty acids as methyl esters. Since the fatty acids are ultimately going to form the basis of the biofuel produced, this is an accurate measure of the total oil yield.
In order for a biofuel process to be successful, it must be sustainable as well as profitable. One measure of sustainability is the amount of CO2 released per unit of energy in the fuel. For biofuels this is reported as the fraction of CO2 released relative to gasoline or another appropriate fuel. But other factors must be considered. These include land usage—especially if land will be taken away from food production or will lead to deforestation—and nutrient usage, including nitrogen and other nutrients. This is especially true for phosphorus, which is believed to be in short supply and whose use could place algal cultivation in competition with food production. Water usage is also an issue, especially if freshwater is used in open ponds and allowed to evaporate. Finally it is important to be able to show that it takes less energy to produce a biofuel than a biofuel can generate.
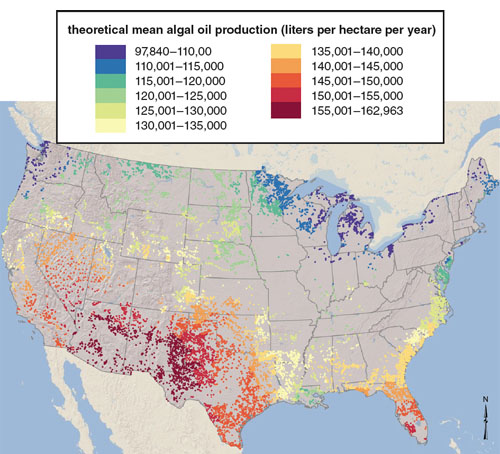
Illustration by Barbara Aulicino. Map from the article “National Microalgae Biofuel Production Potential and Resource Demand,” by Mark Wigmosta et al. 2011. Water Resources Research.
Although these concepts of sustainability may seem obvious, they are notoriously difficult to calculate, as the continuing debate over corn ethanol demonstrates. They are especially difficult for algal biofuels because so many of the values needed for the calculations are available only as estimates or assumptions. Only a few life-cycle assessments have been performed thus far, but the results have shown unpromising energy returns and weak greenhouse-gas benefits. It is vital to get the sustainability calculations right. Huge investments in research, development and deployment are only justifiable with evidence that algal biofuels will be superior to the petroleum-based fuels they may one day replace.
The field of algal biofuels has been criticized because the technical challenges are great and because commercialization is five to ten years away. Nonetheless, significant improvements are being made in all of the technical areas outlined above: algal biology, cultivation, harvest, extraction and analysis. Technoeconomic models have increased in sophistication, and new data are now available to populate models. The best available cost estimates, while high, are becoming more accurate and more useful. These models are reducing uncertainty and quantifying risk, which can give investors more confidence in the likelihood of success in commercialization. With that confidence, more resources from both public and private sectors have been brought to bear on the technical barriers. Although the path to commercialization may be long and may require many millions of dollars, the potential for algal biofuels to contribute to national goals of reduced dependence on fossil fuels, reduced CO2 emissions and greater energy security are worth the investment. We are confident that the barriers will fall.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.