The Latest on Homo naledi
By John Hawks
A recent addition to the human family tree doesn't fit in clearly yet.
A recent addition to the human family tree doesn't fit in clearly yet.

DOI: 10.1511/2016.121.198
The Rising Star cave system, part of the Cradle of Humankind World Heritage Site in South Africa, has been well mapped and was explored by cavers for many years, but without any fossils being noted there. That changed in September 2013, when two South African cavers, Rick Hunter and Steve Tucker, entered a remote, unmapped chamber and found the first-known fossil bones of what is now called Homo naledi strewn across its floor. Hunter and Tucker were working with South African paleoanthropologist Lee Berger, hoping to identify new cave settings with evidence of our fossil ancestors and relatives. Berger quickly organized an excavation to explore the contents of the extremely difficult-to-access site, named Dinaledi Chamber.
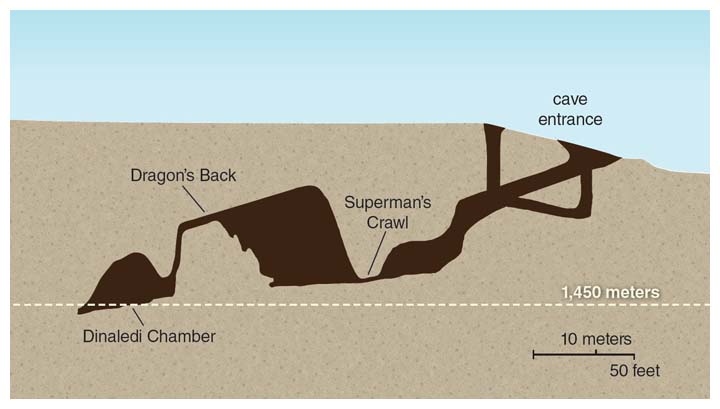
Since the discovery, I have been privileged to be a member of the scientific team investigating the find. The remote chamber would prove to contain a bone bed of an extinct population, more than 1,500 fossil specimens of which have been located so far—one of the most significant finds in the history of studying human evolution. That remarkable discovery has rippled excitement throughout the anthropological community, not least because the species’ traits do not make clear where it fits into the human evolutionary tree.
Our work describing these fossils as the new species H. naledi has been carried out by a large international team of specialists, and the description of the species that we published last year is just the first chapter of a long scientific story.
We assembled a team of scientists to encompass specialists in different aspects of the anatomy of the body, who could contribute extensive data sets and experience to the project. At the same time, we tried to broaden the set of people involved in the description of new hominin fossils, by including members of the next generation. We were able to accept applications from more than 30 early-career scientists, representing 15 countries and 5 continents. The high level of commitment of this team of specialists allowed us to develop an initial understanding of the overall sample within two years of its discovery.
With Berger, I helped organize a symposium to describe our ongoing work at the April meeting of the American Association of Physical Anthropologists in Atlanta. The session gave an opportunity for 13 members of our team, mostly early-career scientists, to present ongoing and unpublished work to colleagues from around the world. We aimed to provide a comprehensive view of the biology of this new species, with presentations covering every part of the body, literally from head to toe. This research begins to address the big questions about H. naledi, but many of those questions still don’t have answers.
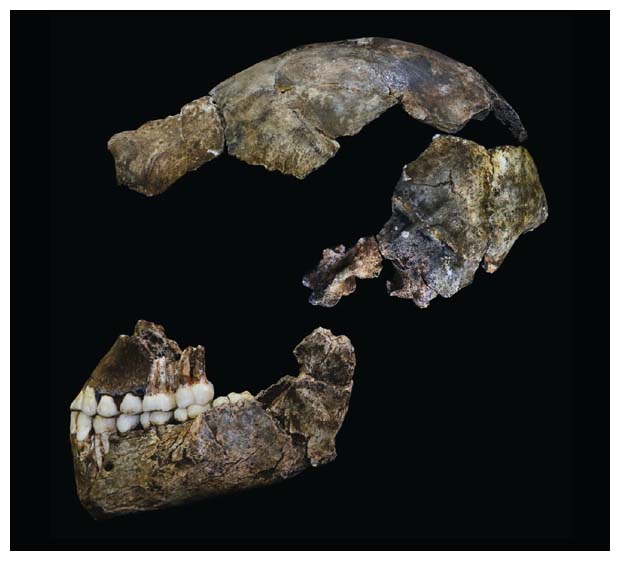
Early in the morning session, team members Lauren Schroeder of the University of Buffalo, Lucas Delezene of the University of Arkansas, and Matthew Skinner of the University of Kent described the teeth and skulls of the H. naledi material. The overall pattern of these specimens is unique, with a confusing mix of seemingly primitive and derived traits. The species has several anatomical details also known in early species of our genus, such as Homo habilis and early members of Homo erectus. But it has a much smaller brain size than is typical of these species. In this and several aspects of its teeth, H. naledi resembles species that branched from our family tree much earlier in time, such as 3.2-million-year-old Australopithecus afarensis, the species of the famous “Lucy” fossil skeleton.
In evolutionary biology, such a mixture of features is known as an anatomical mosaic. Paleoanthropologists have learned over the past century that human evolution was not a gradual progression from a very apelike ancestor to modern humans. Our small canine teeth and more upright posture evolved very early in our lineage, our pattern of bipedal walking next. At the midpoint of our evolutionary tree, ancestors and relatives went on a spree of evolving larger molar and premolar teeth—a trend that turned around when the first members of our genus, Homo, started hunting and making stone tools. Only then did they develop the kind of social sharing that led to language, which today characterizes people around the world. The brain evolved late, the legs early, and every species in our ancestry has its own mosaic of features from this legacy.
H. naledi’s mosaic seems to conflict with this storyline. Nowhere is that more evident than at its hand, arm, and shoulder. Tracy Kivell of the University of Kent reported on the combination of a wrist and fingertips more humanlike than those of H. habilis, combined with very curved fingers, comparable to those of both the very earliest hominins and to living apes. Elen Feuerriegel of Australia National University showed a shoulder canted upward on the trunk where it would suit a climbing species, and an upper arm twisted in a way unlike other human relatives. Both match well to the thorax anatomy considered by Scott Williams of New York University, with a narrowed upper rib cage. H. naledi appears to have been a climber and a possible toolmaker, although we have not yet found any stone artifacts in the Dinaledi Chamber.
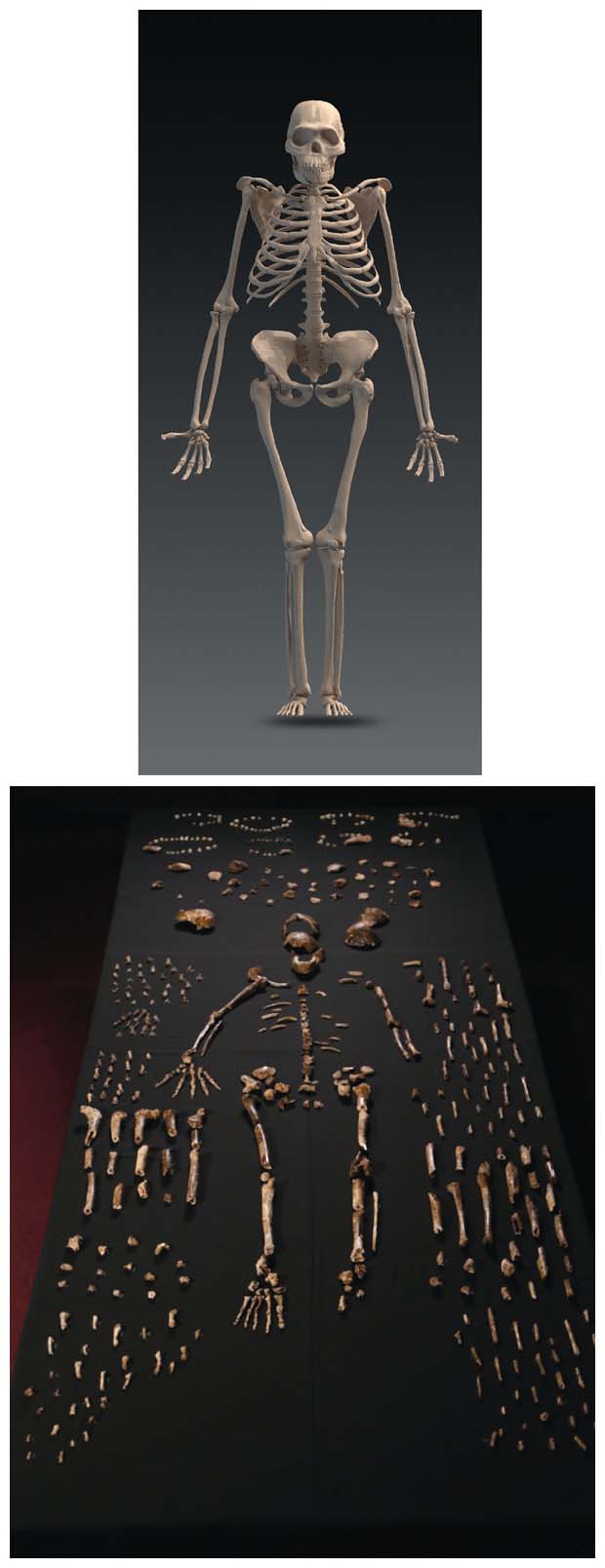
The legs of H. naledi were long and comparatively slender, with some evidence for an elongation of the lower leg, according to Damiano Marchi of the University of Pisa and Christopher Walker of Duke University. However, Caroline VanSickle of the University of Wisconsin–Madison reported that the hips substantially share an anatomical pattern with the much-shorter-statured Lucy skeleton. Zach Throckmorton of Lincoln Memorial University took on the task of building this mixture of features into an overall picture of H. naledi’s gait, noting the evidence for a more humanlike foot.
At the conclusion of the symposium, two talks integrated a broader picture of the biology by examining issues of body size and development. Heather Garvin of Mercyhurst University in Pennsylvania reported that adult individuals of H. naledi weighed between 40 and 55 kilograms, and were between 140 and 160 centimeters in stature, with males and females differing only slightly in size. She emphasized that H. naledi had a very small brain for this size. Debra Bolter of Modesto Junior College in California showed analysis of a range of individuals of different ages, represented by dental remains in the fossil deposit, from infants through very old adults. This sample is exceptional in our evolutionary record for preserving the anatomy of all life stages from one population. Further research on the growth and development of H. naledi may help us to understand how humans have come to have such uniquely long childhood development in comparison with other primates.
The overall picture given by the results presented at the April symposium is that H. naledi walked more or less like humans do, seems to have had hands well made for handling and manipulating objects, and had teeth that indicate a high-quality diet—all things that link the species to our genus. Yet, it had a trunk, hips, shoulders, and fingers that contrast with that picture, and it had a brain similar in size to those of some of the earliest branches of the hominin lineage. In many ways H. naledi was adapted like a human, without anything like a human brain.
Further context for H. naledi’s place in the human lineage may come from the site of its discovery. Marina Elliott, a postdoctoral scholar at the University of Witwatersrand in Johannesburg, reported on continuing work aimed at understanding the context of the fossils. She is now leading excavations at Rising Star, where priority has shifted from the Dinaledi Chamber to other parts of the cave, as we broaden our understanding of how sediments formed throughout the cave. Most other fossil sites in South Africa include the bones of many animals, with hominins being among the rarest. Strikingly, the Dinaledi Chamber contains no other medium or large animals other than hominins. This situation makes it a treasure for understanding their anatomy, but it creates challenges in interpreting the context and age of the assemblage. The team has published data showing that the bones bear no trace of carnivore activity, and that the sediments in the Dinaledi Chamber represent an isolated depositional environment, different from nearby chambers, which were not carried into the chamber by water action. Our best hypothesis is that H. naledi were using the chamber to deposit their dead, but as Elliott noted, we are developing more data to test this hypothesis.
We planned a half-hour question-and-answer session at the end of the symposium session, facilitated by team members Steven E. Churchill of Duke University and Darryl J. de Ruiter of Texas A&M University. It was one of the most interesting periods of open discussion I’ve seen at a professional conference. People offered a broad range of perspectives about the H. naledi fossils, the site, and our open-access philosophy regarding the data. But during the discussion, one question was posed above all others: How will we be able to figure out the age of the fossils?
In many ways Homo naledi was adapted like a human, without anything like a human brain.
The H. naledi analysis was unique in recent paleoanthropology for proceeding on the basis of anatomy alone, without knowing the age of the fossil deposit. This approach was taken partly out of necessity, because of the lack of many of the usual hints regarding geological age. But also, we recognized that the placement of a species into the family tree of organisms, or its phylogenetic position, is one that depends on the pattern of branching in the tree and not the age of the branches. H. naledi ’s anatomical mosaic makes the age determination particularly difficult—did it acquire derived traits early or preserve primitive traits late?
Still, the question of its age is a fascinating one that will test hypotheses about the environmental setting of H. naledi and answer the question of whether it coexisted with any other hominin species. We were able to report that we are working on determining the geological age of the fossil deposit, applying techniques that can be used to date the site’s flowstones (sheetlike formations of calcite that grow where water flows down walls or floors in caves). We are also using some destructive mechanisms to examine the bones and teeth themselves. Developing the chronology is a difficult undertaking, and we are committed to being cautious as we proceed.
Another aspect of the project brought applause from the audience. High-resolution scans of many of the key H. naledi specimens have been posted to the MorphoSource website, where anyone can download them free of restrictions. Churchill reported that thousands of people around the world have downloaded scans for 3D printing, making their own copies of these fossils. At a lecture following the symposium, Berger announced that surface scan data from Australopithecus sediba, another South African fossil discovery, would immediately be available on MorphoSource as well. These technological developments could be quite revolutionary for anthropology.
Moving forward, our team, led by Elliott on site, is excavating a second fossil locality within the cave, hoping to add more information about the overall setting of these hominins. Meanwhile, more investigators have visited the fossil collection to examine other aspects of the biology of H. naledi. We expect most of the work described in the symposium to be published over the next year. —John Hawks
John Hawks is the Vilas-Borghesi Distinguished Achievement Professor of Anthropology at the University of Wisconsin–Madison. He blogs about paleoanthropology at http://johnhawks.net/.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.