How Tunas and Lamnid Sharks Swim: An Evolutionary Convergence
By Robert Edward Shadwick
These fishes diverged millions of years ago, but selection pressures have brought them very similar biomechanical schemes for movement
These fishes diverged millions of years ago, but selection pressures have brought them very similar biomechanical schemes for movement

DOI: 10.1511/2005.56.524
In the early 1800s, the British physician John Davy provided the first insight that tunas were different from other fish. He made measurements showing that tunas were not cold-blooded, as all fishes were assumed to be at that time.
In a paper read to the Royal Society of London in 1835, he reported the remarkable fact that deep muscle temperature in a skipjack tuna (Katsuwonis pelamis) was 10 degrees Celsius higher than the warm tropical water in which the fish was captured. Since then, many investigators have documented warm temperatures in muscle and viscera of other tunas.
Russell House
Sharks of the Lamnidae family, such as makos and whites, are now also known to have regional endothermy. On an expedition to Alaska in 2004, my colleagues Diego Bernal, Jeanine M. Donley and Douglas Syme and I made measurements on the salmon shark (Lamna ditropis), another Lamnidae member, and we recorded deep-muscle temperatures of 26 degrees in fish taken from 6-degree sub-arctic water.
Barbara Aulicino
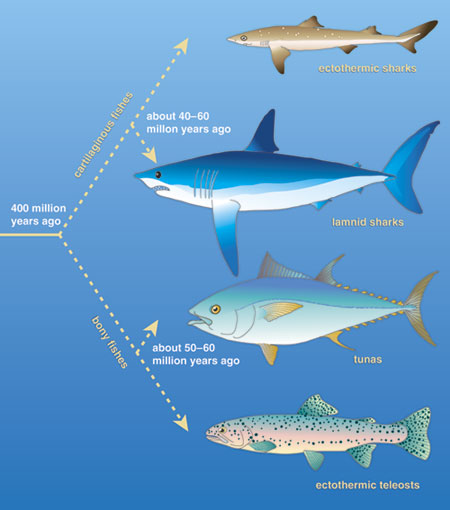
This parallel between tunas and the sharks of this family, called lamnid sharks, is just one of the many anatomical and physiological specializations shared by these two groups of highly active pelagic predators. The evolutionary convergence between them is so striking that in many ways these distantly related groups resemble each other more than they resemble their own close ectothermic relatives. Such similarity is more remarkable considering that these features evolved independently, long after the ancestors of bony and cartilagenous fishes diverged more than 400 million years ago. The shared characteristics in these distantly related groups, which distinguish them from virtually all other fishes, probably arose roughly 40 million to 60 million years ago from similar selection pressures for fast and continuous locomotion.
My colleagues and I have recently made significant advances in quantifying some of these characteristics, particularly the nigh-identical mechanisms of internal biomechanics that evolution has bestowed upon these two groups of fishes. Although tunas and lamnid sharks are not exactly the same anatomically, they have developed systems—to create a hydrodynamic body shape, keep warm and transmit muscle force to the tail—that perform in precisely the same fashion. Our studies have confirmed how tunas and lamnid sharks are able to produce such high levels of power and speed during swimming.
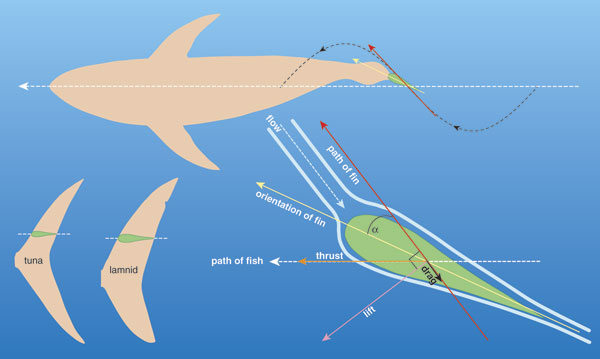
Tunas and lamnids are among the most active apex predators cruising the world's oceans, and both groups have evolved as specialists, in similar ways, for a highly active lifestyle. They can grow to very large size (several meters and hundreds of kilograms), swim constantly for their entire lives and cover long distances in annual migrations. In terms of their anatomy and physiology, tunas and lamnids represent an extreme in biological design for swimming. The groups have winglike pectoral fins and a stiff crescent-shaped caudal, or tail, fin, all of which create lift in much the same way as an aircraft wing, although these fishes apply the force to forward thrust instead of upward lift. Both tunas and lamnids have robust, muscular bodies that are so well streamlined that they are nearly ideal for drag minimization in steady swimming. In fact, the name for this teardrop-shaped body in fish, and the swimming mechanism they use, is thunniform, from Thunnus, the genus name of many tunas.
Barbara Aulicino
This shape positions the bulk of muscle in the thick mid-body region, and pronounced tapering results in a caudal peduncle—the region where the tail attaches to the body—that is very narrow and flattened with lateral keels, giving a shape that reduces drag as it moves side to side in propulsive tail strokes. In tunas, body streamlining is so complete that during high-speed swimming, the dorsal fin retracts completely into a groove on the back, and the pectoral fins can fold flush against the body by fitting into surface depressions—features that are unique to tunas. Both tunas and lamnids have body densities greater than seawater, making them negatively buoyant. This means they must swim constantly, but it is an advantage during feeding bouts: It allows rapid movements from warm surface waters to cold depths where the animals hunt their prey. Some tunas and lamnids have been reported to reach speeds, in short bursts, of over 15 meters per second.
The similarity between these predators is more than skin deep. Internally there are also impressive and surprisingly parallel adaptations in anatomy and physiology. It is likely that streamlining is at the root of some of the underlying anatomical specializations, and other internal adaptations are directly associated with high-performance locomotion in these fishes.
Barbara Aulicino
In particular, there are enhancements to the biological machinery to support the elevated metabolic rate that powers continuous aerobic swimming: Oxygen transfer at the gills and delivery to active tissues is facilitated by a large heart pumping blood through gills that themselves have a much-increased surface area and very thin gas-exchange barriers. These fishes must swim with their mouths partially open all the time to provide sufficient water flow over the gills, in a process called ram ventilation. Blood pressure and cardiac output are also increased, approaching the levels that are achieved by mammals. Even the concentration of hemoglobin in the blood, which determines the oxygen-carrying capacity, is elevated compared to other fishes; furthermore this hemoglobin is specialized to perform well at the elevated core temperatures that tunas and lamnids maintain. This increased capacity to deliver oxygen and nutrients to the muscles lets these fishes sustain high levels of such activities as continuous swimming.
Interestingly, the heart is not kept warm like the core muscle and viscera, so it faces the challenge of powering a high-pressure circulatory system while operating at variable and often cold temperatures. Again, tunas and lamnids have special anatomical and biochemical features, not found in other fishes, which specifically allow their hearts to be effective at low temperatures and to support high metabolic demands. Barbara A. Block and her colleagues at Stanford University have shown that hearts in tunas living in cold seas have increased quantities of sarcoplasmic reticulum, the intracellular membrane structure that enhances storage and release of calcium ions in heart cells. Calcium ion release is an essential event in each contraction-relaxation cycle of heart muscle, and this increase may allow the heart to beat faster at low temperature than the hearts of ectothermic fishes. Block's group has also shown that the salmon shark heart has elevated levels of Ca2+-ATPase, an enzyme that actively pumps calcium ions into the sarcoplasmic reticulum, presumably conferring high cardiac performance at low temperatures.
A hallmark of lamnids and tunas are the elaborate blood-vascular retia, a structural modification of circulatory vessels that allows retention of the muscle heat generated from continual contractions. Incoming and outgoing vessels lie close enough to each other that heat from warm venous blood can be transferred to cooler arterial blood that is flowing into the muscle region. This counter-current heat exchange results in the elevated core temperatures found in tunas and lamnid sharks.
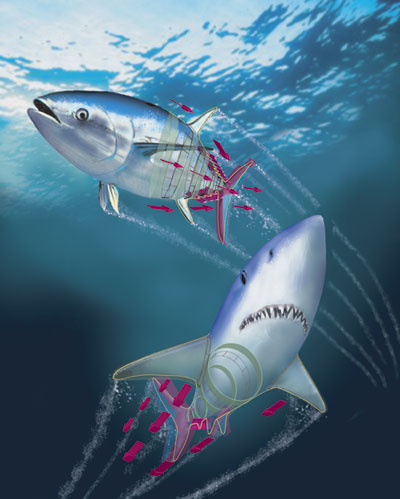
Endothermy is further linked to another internal adaptation found almost exclusively in these two groups. In all fishes, white muscle fibers are anaerobic and power short-burst swimming, whereas red muscle fibers are aerobic and sustain constant swimming. White muscle is formed in layers, called myomeres, which are folded in a sideways Wshape around the backbone of the fish, with their points nested together. In virtually all non-endothermic fishes, the red muscle forms a thin wedge just under the skin, on top of the myomeres—but in tunas and lamnid sharks, it is found as a loin medially, deep in the body. Furthermore, the red muscle is concentrated more forward in the thickest part of the body, with the peak in its cross-sectional area occurring at about the level of the main dorsal fin. The muscle's deep position is what allows the retial heat-exchange system to be effective in maintaining an elevated temperature.
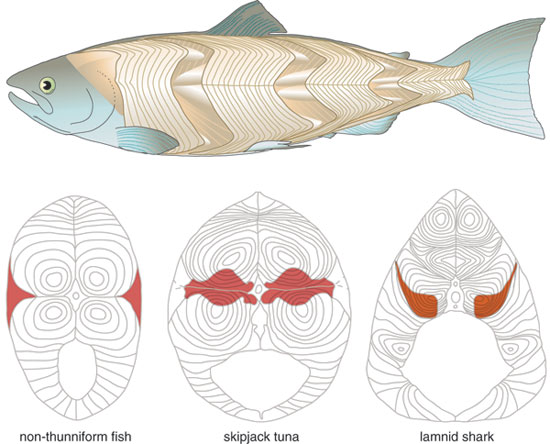
Barbara Aulicino
This anterior and medial positioning of the aerobic muscle mass might at first glance seem to be disadvantageous for powering continuous swimming: the muscle is far from the tail, which produces most of the propulsive force. It is also close to the bending axis of the spine, whereas a position just under the skin would seem to allow it to do more work, much like increasing the length of a lever. But instead this red-muscle placement turns out to provide substantial benefit; in fact, this anatomical specialization provides the basis for the thunniform swimming mode. In order to appreciate how this works, it helps to consider how other fishes swim.
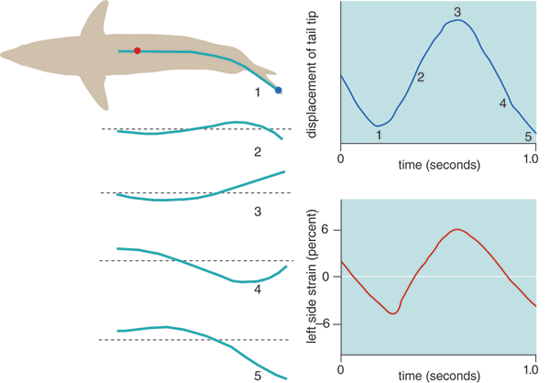
The majority of fishes swim forward by undulating, generating a propulsive wave of lateral bending that travels backward along their bodies. They do this by sequentially contracting muscle segments alternately along both sides. The motion of this wave is difficult to see with the naked eye, but with slow-motion film or video, it can be readily characterized. Each portion of the body involved in propagating the wave accelerates water backward and sideways, producing a reaction force of which there is a forward component that contributes to thrust, but also a lateral component that wastes kinetic energy and makes the head recoil sideways. Additionally, body undulation increases viscous drag significantly compared to the same body in a straight posture. Typically the body wave increases in amplitude from head to tail (the lateral displacement at the tail tip is typically 20 percent of body length), so the more-posterior portions of the body have a greater angle of inclination toward the direction of overall movement, move laterally at higher speeds and generate higher reaction forces. Clearly, an efficient swimming mode would maximize thrust while minimizing these lateral and drag forces.
In non-thunniform swimmers, muscle contractions along the body are transmitted directly, or coupled, to the adjacent vertebrae and therefore result in bending of the local body region. Thus a progressive increase in proportional shortening (or strain) of muscle is needed to create the increased lateral displacement in the more-posterior regions of the body. The relation between strain amplitude and the amount of bending it causes is dependent on the distance the muscle fibers are from the backbone, or their effective mechanical advantage—again, think of a lever. In a non-thunniform fish's steady swimming, it is the thin subcutaneous layer of red muscle fibers that powers the undulatory motion, and strain amplitudes range from as low as 2 percent in the anterior to up to 6 percent in the posterior. These are relatively modest when compared with strains of 20 to 30 percent typically achieved in the skeletal muscle of flying and running vertebrates.
Since contractile work and power are determined in large part by the strain (work is force multiplied by length change, power is the rate of work production), one might expect that muscle strains should be high to maximize performance. Indeed, fish muscle is capable of such high strains, and these do occur in extreme turns or fast starts when the body is thrown into much greater curvature than seen during steady swimming. So why is muscle shortening so limited in the routine swimming of non-thunniforms? One obvious answer is that although lateral motion is needed to generate thrust, it is also has the negative consequences previously described—adding to drag and wasted energy. Thus, the fish is caught in a paradox: Higher muscle strains could increase power output but at the same time would disproportionately increase the power required to swim because of greater body bending, drag and kinetic-energy costs. The compromise to solve this paradox is to generate a bending wave that has modest lateral amplitude over much of the body, at the expense of high degrees of muscle shortening and power production.
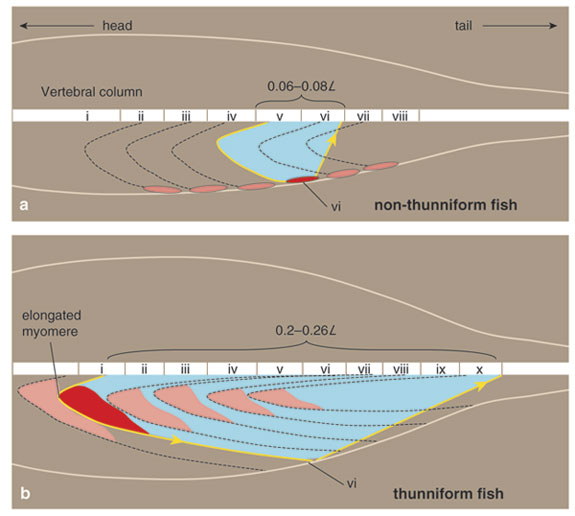
These muscle-strain levels would be expected to be even lower if the red muscle was situated against the backbone, as it is in lamnids and tunas. But if the thrust production could be focused into the tail, and if muscle residing in the main portion of the body could be used to move the tail by some linkage elements instead of causing local bending, then muscle strains could be increased even while lateral motion was restricted to the tail, thus saving energy. It turns out that this is what tunas and lamnids have actually done as they independently evolved thunniform locomotion systems: They use their anteriorly shifted muscle mass to power swimming, while also reducing lateral motion of the mid-body region. Thus, the red muscle placement deep in the body, where it can stay warm and therefore contract more quickly and powerfully, does not decrease the muscle's mechanical advantage, as the force is being applied much farther down the body.
This transfer of muscle power to posterior regions of the body is accomplished using a unique and defining physical feature shared by tunas and lamnids: Their red-muscle loins are physically disconnected from the surrounding white-muscle sheets by a sheaf of loose connective tissue, and the contraction of the red muscle is also uncoupled from local body bending, instead being transmitted toward the tail through a series of long tendons. The details of this novel biomechanical design and the extent of the convergence between lamnids and tunas have come to light during kinematics studies by my research group, until recently at the Scripps Institution of Oceanography, and parallel anatomical investigations by Sven Gemballa and his colleagues at the University of Tübingen in Germany.
Our first studies were conducted at the Kewalo Tuna Research Facility in Honolulu with Torre Knower and Stephen Katz. We swam live skipjack and yellowfin (Thunnus albacares) tunas at controlled speeds in a large treadmill flume—the aqueous equivalent of a wind tunnel. Thus, the fish swam actively, but were stationary, due to the strong oncoming current. We quantified body kinematics from videography, while muscle activity was measured simultaneously with fine wire electrodes implanted in muscle. In the initial tuna measurements, we assumed strain of deep red muscle was coupled to local midline curvature, as it is in other fishes. The results were paradoxical: We found that muscle activation all along the body seemed to occur too late, well after bending (and inferred shortening) at each location began.
Barbara Aulicino
But in subsequent tests, we measured the strain of the deep red muscle directly by sonomicrometry, where probes inserted into muscle emitted ultrasound pulses during muscle contraction in active swimming. These measurements demonstrated that, indeed, shortening of tuna deep red muscle in each tailbeat cycle starts significantly later than changes in local curvature, such that shortening in mid-body muscle is actually in phase with the curvature eight to 10 vertebral segments (or 15 to 20 percent of the body length) farther back. Furthermore, strain amplitude of the deep red muscle is considerably larger that predicted from the curvature—six percent as opposed to three percent predicted at mid-body. We realized that this muscle is physically uncoupled from local body curvature, and therefore its close proximity to the backbone does not, as might be expected, bestow a mechanical disadvantage that would limit its ability to shorten and produce power. We also found that the activation of red muscle does begin just before the muscle reaches its peak length and continues well into shortening, as is typically observed in muscles that are optimized for contractile work output.
In further experiments Syme and I examined the contractile mechanics of deep red muscle using a process called the work-loop technique. This is an in vitro approach that takes bundles of live muscle fibers in physiological saline and induces them to contract cyclically using an apparatus that can control strain amplitude, cycle frequency and stimulation timing, in order to determine how these parameters affect the work produced by the muscle. In each contraction, the length change imposed by the apparatus and the subsequent force generated by the muscle are recorded. The integral of the force signal with respect to the length signal is a measure of the work done in the contraction cycle. The work per cycle is then multiplied by the cycle frequency to give the power output. By simulating actual contraction conditions, determined from in vivo experiments, we can estimate the muscle's performance during swimming. Our results showed that the activation patterns that are observed in vivo yield maximal muscle power output at both the anterior and posterior ends of the overall body musculature. In other words, all aerobic muscle along the body is involved in producing positive thrust power, highlighting the specialization of tunas for continuous and steady locomotion.
Barbara Aulicino
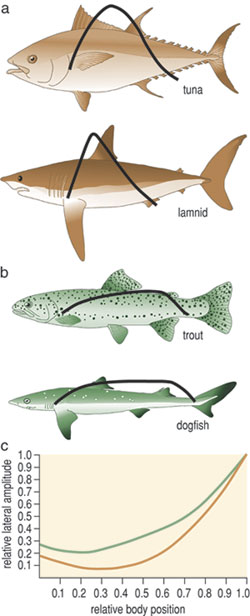
Non-thunniform, undulating swimmers lack this uniformity in muscle use. In fish such as bass, the posteriorly placed muscle delivers the majority of the thrust power because it undergoes the largest strain amplitude—the undulatory wave of muscle contraction increases from head to tail in these fish. There are, however, much smaller contributions to thrust from the muscle in the anterior and mid-body regions, where strain amplitudes are lower.
A significantly different situation occurs in other undulating swimmers, such as trout or carp, as these fishes have regional variations in muscle function. While some of the anterior musculature is recruited to produce most of the thrust power, the more posterior regions are also involved in stiffening the body by performing so-called "negative work" during part of each contraction cycle. This occurs if a muscle is activated while being lengthened, instead of when it's about to start contracting. In this situation, the muscle absorbs energy in the same way as a tendon does when it is being stretched. Some investigators have hypothesized that such muscle-activated stiffening also occurs in tuna, and accounts for their thunniform swimming style, but our studies prove that this is not the case. The uniformity of muscle function along the body of tunas (and lamnids) can be viewed as another way in which these fishes are optimized for locomotion.
In a swimming tuna, muscle activation and shortening also travels along the body very quickly, so that the whole loin of red muscle contracts nearly simultaneously, rather than in a discernable traveling wave, as is typical in other fishes. The consequence of this design can be seen when the muscle and body kinematics are compared. Lateral motion of the thrust-generating tail is driven by muscle contractions from mid-body and posterior. If the head of the fish is taken to be position 0, and the tail position 1, then when red muscle at about 0.4 of body length on one side begins to shorten, the tail begins sweeping to that side. Shortening at more posterior locations is only slightly later, so the caudal propeller is driven by red muscle fibers acting nearly in parallel.
Based on the similarities in morphology and swimming mode, my colleagues at Scripps, including Donley and Chugey Sepulveda, and I employed the same techniques that we had used with tuna to study small mako sharks (Isurus oxyrinchus) swimming in captivity. As in our investigations of tunas, we found that the anterior and medial placement of the red muscle has the same consequence, for the same reason, in makos. The myomeres are highly elongated, and the red fibers are located internally on the cone tip, so this results in a significant anterior shift in muscle location as well. Red-muscle shortening is also directed to the posterior body region and, as in tunas, this projection extends down about 20 percent of the body length. Thus, the similarities in mechanical design are remarkable, even to the extent of comparable changes in muscle anatomy.
In particular, the deep red-muscle fibers of the mako appear to function with the same physical uncoupling from local body bending as found in tunas, but the uncoupling is slightly more pronounced in the more-posterior locations. In slow-swimming makos, deep red-muscle contractions in the mid-body region are delayed by about 10 to 15 percent of a tail beat period relative to the surrounding inactive white muscle and the local curvature; therefore shortening at 0.4 of body length is in phase with midline curvature at about 0.6 of body length, while shortening at 0.6 of body length is nearly half a cycle out of phase with local curvature. As with tunas, activation timings recorded in vivo match those that produce maximal power in anterior and posterior red muscle, based on work-loop experiments, thus demonstrating the specialization of these sharks for continuous swimming. In addition, the activation and muscle-strain waves travel along the body relatively quickly, and the whole red-muscle loin shortens nearly in synchrony to create the lateral motion of the caudal fin.
Clearly, the major feature underlying the biomechanical convergence of tunas and lamnids is the unique muscle-tendon architecture that facilitates their thunniform swimming mode, allowing red muscle in the mid-body to cause large amplitude lateral motion in the caudal region. Recent studies by Sven Gemballa and Peter Konstantinidis at the University of Tübingen suggest that in ectothermic fishes, the limitation of red-muscle action lies in the arrangement of the tendons that link the superficial red muscle to the axial skeleton. They have shown how architectural alterations in these linkages allow tunas and lamnids to project contractions of their internalized red muscle to the caudal region. This long-reaching force-transmission system is facilitated by extreme myomere elongation, including the lengthening of tendons that join to the myoseptum, the connective tissue between myomeres. But most important, situating the red muscle fibers deep in the anterior-pointing myomere cones means that they can attach to tendons that are not only unavailable for the superficially-positioned fibers in other fish, but also can span large numbers of body segments—enabling the fish to project muscle force toward the tail.
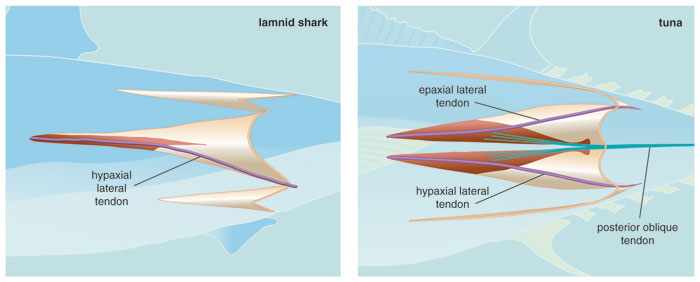
Data for illustration courtesy of Sven Gemballa, University of Tübingen; illustrated by Barbara Aulicino.
Despite this convergence in the musculotendinous design of tunas and lamnids, there are some differences in the red muscle-tendon linkages. In tunas, deep red muscle attaches to the anterior portion of two tendons, termed the anterior cone tendon and the lateral tendon. These are both present in the upper and lower quadrants on each side of the body. These cone tendons join to tendons in the horizontal septum that are directed obliquely to vertebrae in a more-posterior position. This is in contrast to the arrangement of superficial red-muscle fibers in cold-blooded teleost fishes, which join with the posterior portion of much shorter lateral tendons and link into oblique tendons with steeper angles to the vertebrae, thus spanning much shorter axial distances. A second pathway of force trajectory from deep red muscles in tunas is via myoseptal lateral tendons, which run from anterior to posterior myomere cone tips. Again, due to their deep location, red-muscle fibers insert onto the anterior portion of these long lateral tendons, which then direct force to the rear via subsequent posterior cone linkages. In the most posterior myomeres of tunas, the anterior cone tendons and lateral tendons coalesce to form robust tail tendons that insert directly into the caudal fin.
The arrangement is much the same in lamnid sharks, with a few distinct differences. The anterior myomere cone is not bifurcated in lamnids, so red muscle inserts only onto hypaxial (or ventral) lateral tendons rather than both epaxial (dorsal) and hypaxial lateral tendons. Internalized red muscle connects to the anterior portion of these tendons, which then project caudally through white muscle to the posterior cones and finally into skin in the region of the peduncle. Lamnids, unlike tunas, have no equivalent of the horizontal oblique tendons. Instead, it is presumed that collagen fibers in the skin then act as the final segment in this force-transmission system and project the muscle forces to the tail fin. Although the hypaxial lateral tendons are involved in force transmission in both groups, the remaining tendons in tunas result in a more dorsoventrally symmetric musculotendinous design.
Although these two groups of fish may not have precisely the same internal anatomy, the overall function of their biomechanics is virtually identical and separates these two groups from nearly all other fishes. It is true that, in addition to the lamnids and tunas, a thunniform body shape has also evolved in other aquatic cruising specialists, such as dolphins, whales and extinct ichthyosaurs. This suggests that hydrodynamic demands have provided important selection pressures to optimize body shape for locomotion in the course of vertebrate evolution. However, no other ocean dwellers, no matter how streamlined, have had their internal biomechanics taken to such an extreme of specialization as that found in tunas and lamnid sharks. That evolution has reached this endpoint twice, in separate circumstances, is a testament to just how efficient this body design is, both inside and out, for high-performance swimming.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.