Herschel and the Puzzle of Infrared
By Jack R. White
An astronomer took a mental leap to first connect light and heat.
An astronomer took a mental leap to first connect light and heat.

DOI: 10.1511/2012.96.218
Most encyclopedias and physics books credit the great British astronomer Sir William Herschel with the discovery of infrared radiation in 1800. It’s a good story, but it is not strictly correct—it trivializes the true significance of what Herschel found.
We all discover infrared at a young age when we feel warmth at a distance from a hot object, and we know that these rays are invisible—warmth can be felt in total darkness. What Herschel discovered was subtler than the existence of invisible radiation. He found the first solid evidence that light and infrared are the same quantity that we know today to be electromagnetic radiation. Through a series of simple experiments, Herschel found the first piece in one of the great puzzles of physics that took another century to solve.
Photograph courtesy of the author.
The best information about Herschel’s experiments is found in his original papers. His recorded data and many of his comments appear to have been taken directly from his lab notes, and their freshness and authenticity come through even today. The biggest challenge reading his work is to follow his line of reasoning through many digressions and pages of raw temperature data.
In a paper read before the Royal Society on March 27, 1800, Herschel called this warmth felt at a distance “radiant heat.” This description is still a good working term for infrared radiation. The term “infrared” did not enter scientific vocabulary until the 1880s. Infra is Latin for “below,” but researchers have been unable to trace the source of who initially coined the name.

National Portrait Gallery, London
Herschel was not originally a scientist. He rose from obscurity as a German immigrant in a military band to become an accomplished musician and composer. In 1773, at age 34, six years after moving to Bath to take a position teaching music and playing in concerts, he did something that changed his life and fortunes. He bought a small telescope and a book on astronomy.
By the following year, he was grinding his own mirrors to build larger and better quality telescopes, and spending his nights studying the heavens. Herschel’s craftsmanship rapidly took his hobby to public recognition as the foremost telescope manufacturer of his time. His fame peaked after he discovered the planet Uranus in 1781, which led to his appointment as the king’s astronomer.
Following his appointment, which came with an annual salary of £200, Herschel was able to devote all his time to astronomy. He and his sister, Caroline, settled in the town of Slough, near Windsor Castle. A condition of his appointment was that he be available to King George III and the royal family any time they wished to view the stars.
Herschel’s foray from astronomy to infrared was a fortuitous tangent from his effort to find the best color for a filter that would allow him to safely view the Sun. His speculations and conclusions were often contradictory: Many were wrong, but some were extraordinarily prescient. The story of Herschel’s experiments is that of the role of human perception in scientific discovery, as well as the conflict between conventional beliefs and concepts never before encountered.
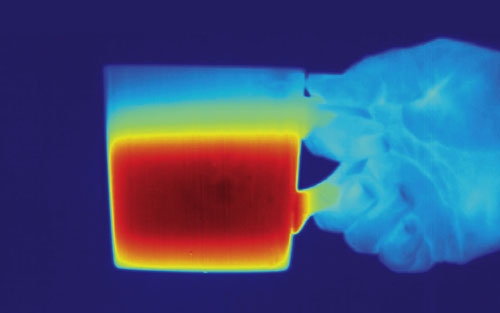
The vast electromagnetic spectrum stretches from gamma rays (whose wavelengths can be smaller than the width of an atom) to radio waves (whose wavelengths can reach thousands of kilometers). Of this range, humans are only able to directly sense radiation in two small bands. Our eyes see light, which occupies a narrow sliver of wavelengths from 0.4 to 0.7 micrometers, centered approximately where the Sun’s radiant power is at its maximum. Our skin feels warmth mainly from infrared, which spans the range of wavelengths between light and microwaves, up to about 1,000 micrometers.
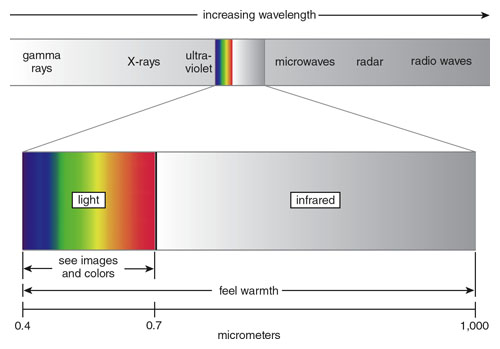
The boundary between light and infrared is determined by the long-wavelength limit of the human eye’s response. Everyday experience would not lead us to believe light and infrared are the same kind of energy. Indeed, two compelling pieces of evidence suggest, logically, that they are not related.
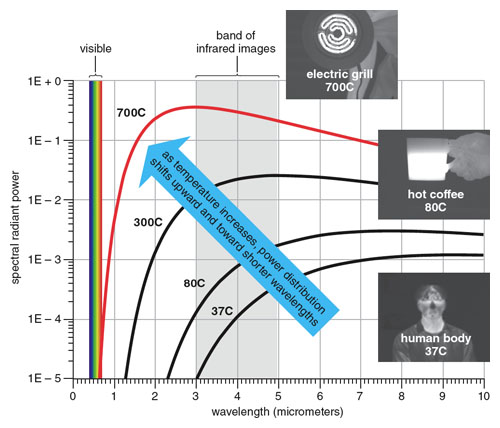
First, we experience light and infrared differently with different senses. We see light, perceiving different wavelengths as different colors, but we feel infrared only as warmth. Second, light and infrared aren’t always found together. Most sources of light also emit infrared, but infrared is often found by itself. A familiar example is an electric grill not hot enough to glow: If the room is completely dark, we can still feel the warmth from the grill, but we can’t see it.
Photographs courtesy of the author.
These differences may be why the connection was not made for so many centuries in spite of much experimentation. Perhaps this counterintuitive association is why the connection was found almost by accident by a person with no formal scientific training.
Herschel had observed features on the Sun’s surface for a number of years, presenting a paper on the Sun and fixed stars to the Royal Society in 1794. Being able to observe sunspots with a large telescope without damaging his eyes had long been a challenge. Through experiments with different combinations of colored and darkened glass, Herschel observed, as he noted in this paper:
What appeared remarkable was, that when I used some of them, I felt a sensation of heat, though I had but little light; while others gave me much light, with scarce any sensation of heat.
This observation led to the thought that different colors might, in Herschel’s words, “have the power of heating bodies very unequally distributed among them.” Herschel further reasoned that if the heating power were unequally distributed, the illuminating power might be as well. There might be a single best color for seeing, and it might be different than the one for maximum heating. Knowing these qualities would help him find the best filter to view the Sun.
Drawing on his experience making telescopes, Herschel built an instrument to test his hypothesis. He made what we would call a spectrometer, or more precisely, a spectroradiometer: an instrument to measure the magnitude of radiant power at different wavelengths.
His first instrument consisted of three components: a prism set in a south- facing window to catch the sunlight and direct and disperse the colors down onto a table; a small panel of cardboard with a slit wide enough for only a single color to pass through; and three mercury- in-glass thermometers (of which he used two) with their bulbs blackened to better absorb light. Thermometers were not common household items in 1800, but Herschel had one of his own and borrowed two more from a colleague.
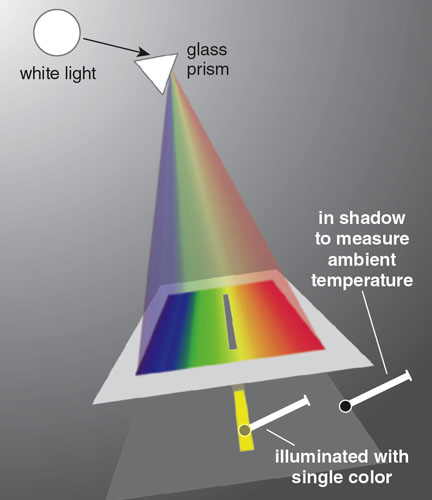
He placed one thermometer in the light and kept the other two in darkness to measure the room’s ambient temperature. Herschel understood there were, as he expressed it, “causes acting in different ways” (in other words, conduction and convection) that affected the stabilization temperature of the thermometers, and he wanted to quantify the heating caused by the light alone.
Spectrometers today have much higher resolution, greater sensitivity and faster response, but the basic functional elements are the same as Herschel’s. Prisms are still used, but better resolution is usually obtained through wave interference —wavelengths are separated by constructive and destructive interference, where their waves either add together or cancel out. The detector today would be a cryogenically cooled semiconductor—much smaller, faster and more sensitive than a mercury thermometer. But Herschel’s instrument, in the hands of the careful experimenter that he was, gave surprisingly accurate measurements.
With his device set up on a sunny day, Herschel methodically took temperature measurements, first comparing the thermometers’ readings at ambient conditions to ensure baseline agreement. It was cold in the room. His starting temperatures averaged 43.5 degrees Fahrenheit. After some experimentation, he settled on using his own thermometer with its half-inch-diameter bulb exposed to the light and used the larger of the borrowed ones as the ambient reference.
Herschel positioned the measuring thermometer in the band of colored light for each reading. At each color position, he allowed the thermometer to stabilize for 10 minutes before taking a reading. He took a series of measurements, starting with red, which gave an average reading of 67/8 degrees above ambient. Green gave 31/4, and violet light gave a 2-degree average increase.
From these data, Herschel felt that he had proven his hypothesis about heating being unequally distributed and could move on to the illumination experiment. As he stated in his initial conclusion:
Which only goes to prove, that the heating power of the prismatic colours, is very far from being equally divided, and that the red rays are chiefly eminent in that respect.
To find the maximum of illumination, he directed colors onto a variety of small objects that he viewed through a 27-power microscope. From the brightness and clarity of what he saw, he judged the relative illumination.
The illumination experiment also went well. He did 10 separate experiments with objects viewed in different colors. He attempted to distinguish between the color having the maximum of illumination and that having the sharpest resolution or “distinctness.” He was unable to reach a conclusion about resolution, but for illumination, he was able to state:
The maximum of illumination lies in the brightest yellow, or palest green. The green itself is nearly equally bright with yellow; but, from the full deep green, the illuminating power decreases very sensibly.
This observation is remarkable: Yellow-green is near the wavelength where the Sun’s radiant power is a maximum and is exactly where the eye’s sensitivity is greatest.
Feeling he had proven that both radiant heat and light are not equally distributed across the colors, and with measurement results showing their differences, Herschel should have been ready to move on to applying these results to his problem of viewing the Sun. But he didn’t do so immediately. Instead, he returned to the temperature data.
Something about the temperature readings clearly bothered him. He had expected, as he found, that the readings would be different for the various colors. But the measurements also showed something he did not expect: a trend, rather than a peak.
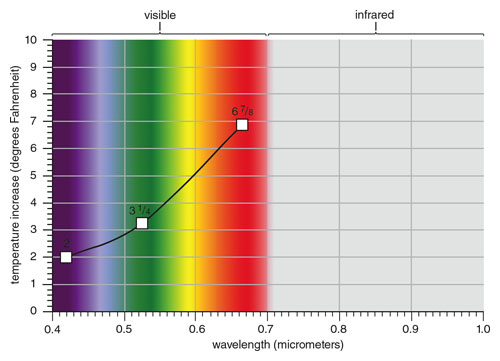
The heating was greatest in red, but the curve did not appear to reach a maximum in the visible spectrum. Instead, the readings seemed to point somewhere in the dark region beyond red. He felt compelled to follow this trend.
If the maximum lay outside the visible spectrum, then the heating was not from light but from something else. Herschel used the expression “invisible light,” cautiously phrasing it in a way that indicated he knew it to be an oxymoron. (If rays are invisible, then they aren’t light.) As he expressed it:
I likewise conclude that the full red falls still short of the maximum of heat; which perhaps lies even a little beyond visible refraction. In this case, radiant heat will at least partly, if not chiefly, consist, if I may be permitted the expression, of invisible light; that is to say, of rays coming from the sun, that have such a momentum as to be unfit for vision.
By describing the rays in terms of momentum, Herschel was not anticipating the discoveries of quantum physics, still a century in the future. Photons at infrared wavelengths do have less energy than those in the visible band. As a result, they do “have such a momentum as to be unfit for vision.” He was not looking a century ahead but a century back—to Isaac Newton’s experiments with light in the late 1600s.
Herschel accepted without question Newton’s beliefs on the “corpuscular” nature of light. A strong case for light being a wave had been made by Newton’s contemporary, Christiaan Huygens, but the theory of light as streams of minute particles dominated science at the time, especially in Britain. This viewpoint changed within the next 15 years, but at that moment, Herschel thought of light as particles that had more or less “efficacy” in their effect on matter.
It wasn’t the concept of invisible rays that so interested Herschel. What captivated him were the properties of these rays. It was clear to him that radiant heat had the same optical properties of “refrangibility” (“refraction” in modern usage) and dispersion as light.
Refraction is the change in direction of a ray as it enters or exits a transparent medium that causes a change in velocity, such as between air and glass. Dispersion is the effect of refraction on multiple wavelengths, causing different rays to refract at differing angles. We see the effects of the dispersion of light most commonly from rainbows and prisms. Herschel didn’t think of light in terms of wavelength, but as a lens-maker, he was very familiar with the effects of dispersion and how to correct for it to produce lenses that minimize what is known today as chromatic aberration, where different colors converge to a focus at varying distances from the lens. As he wrote:
I must now remark, that my foregoing experiments ascertain beyond a doubt, that radiant heat, as well as light, whether they be the same or different agents, is not only refrangible, but is also subject to the laws of dispersion arising from its different refrangibility.
The telescope designer in Herschel grasped the significance immediately: If light and radiant heat have the same optical properties, if they exhibit the same behavior in their interactions with matter, might that indicate they are the same quantity? He notes: “
May not this lead us to surmise, that radiant heat consists of particles of light of a certain range of momenta, and which range of momenta may extend a little farther, on either side of refrangibility, than of light?
It was a question that dominated Herschel’s thoughts and effort for much of the rest of the year. He must have worked rapidly, because just 9 days after writing his first paper and 10 days before he formally presented it, he wrote a second, shorter paper to the Royal Society titled “Experiments on the Refrangibility of the Invisible Rays of the Sun.”
The title of this paper has an intriguing echo of Newton. In Newton’s Opticks (1730), his Proposition II, Theorem II is titled “The Light of the Sun consists of Rays differently Refrangible.”
Newton clearly had great influence on Herschel. The latter’s approach was nearly identical to Newton’s experiments using a prism placed in a window to project colors onto a wall. Herschel took Newton’s basic qualitative method of viewing the spectrum and turned it into a quantitative instrument. He may have felt—justifiably—that he was continuing the work Newton had begun with the colors of light by extending the concept of “different refrangibility” to the rays of the Sun that lay beyond the visible colors.
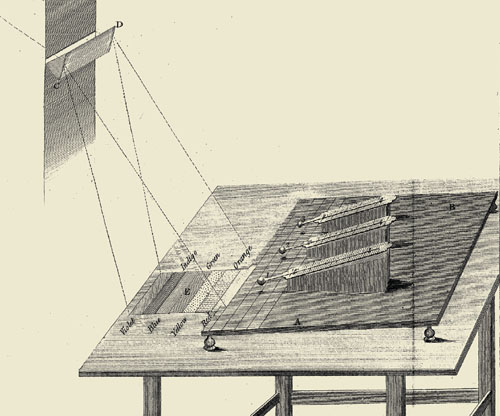
Herschel began his second experiment by slightly modifying his spectrometer to take temperature readings into the dark area on his board, beyond red. His only reference was in relation to where the colors fell on the table. He marked off five parallel lines spaced half an inch apart on a sheet of white paper, with the first line at the edge of the band of red light. Thus anchored to the edge of the visible, Herschel ventured into the darkness beyond.
He took readings with his thermometer, following the upward trend to a maximum and beyond, until the heating began to diminish. The tone of Herschel’s second paper is one of excitement and confidence in his findings. Throughout his experiments, he ventures few opinions that are not firmly supported by data, but he concludes this paper with an argument based on philosophy:
To conclude, if we call light, those rays which illuminate objects, and radiant heat, those which heat bodies, it may be inquired, whether light be essentially different from radiant heat? In answer to which I would suggest, that we are not allowed, by the rules of philosophizing, to admit two different causes to explain certain effects, if they may be accounted for by one.
Herschel’s reputation as an astronomer probably helped ensure that his papers were favorably received by most scientists, but not by all. His third paper opens on a decidedly defensive note. He appears to have been attacked by a person he refers to as a “celebrated author,” who took offense at the phrase “radiant heat.” This detractor may have been John Leslie, who was considered an authority on heat and clearly resented the intrusion of an amateur into his domain. In a letter published in A Journal of Natural Philosophy, Chemistry, and the Arts by William Nicholson, Leslie wrote:
It would appear this able astronomer, on entering a new line of enquiry, has neither employed apparatus suited to the nicety of the subject, nor guarded sufficiently against the numerous and latent sources of error. I consider myself entitled to speak with the greater confidence because I had long directed my researches in the same channel.… I do not hesitate, therefore, to maintain that Dr. Herschel’s capital proposition originates in fallacious observations.… And whatever my sentiments were respecting the validity of the conclusions, I resolved calmly and impartially to subject the pretended facts to the test of experiment. When a photometer was placed beyond the spectrum, … no effect whatever was perceived.
If Leslie’s differential thermometer (he called it a “photometer”) found no heating beyond the visible as he claimed, then it was badly in error. This point was later proven dramatically by independent experiments conducted by the Royal Society. To deflect criticism, Herschel switched from the term “radiant heat” to “the rays that occasion heat.”
Light was also a contentious issue. Herschel’s brash assertions about radiant heat and light had stepped on the toes of conventional belief. With light, he again adopted a cautious stance, but this time he countered with a challenge calculated to silence his critics:
I must also remark, that in using the word rays, I do not mean to oppose, much less to countenance, the opinion of those philosophers who still believe that light itself comes to us from the sun, not by rays, but by the supposed vibrations of an elastic ether, every where diffused throughout space; I only claim the same privilege for the rays that occasion heat, which they are willing to allow to those that illuminate objects.
The criticism he received did not slow his experiments, but these attacks may have had an impact. By the second part of his final paper, his emphasis changed from finding evidence supporting the similarity between light and radiant heat to that supporting their difference.
Herschel’s third paper proposed seven comparisons between light and radiant heat. The first concerns two human senses. The next five are interactions with matter that were known in 1800: reflection, refraction, “different refrangibility” (dispersion), transmission through “diaphanous bodies” (transparent media) and scattering from rough surfaces.
The paper’s final proposition asks whether radiant heat, if sufficiently strong, is able to stimulate vision. This question is critical because the complete answer explains why light and infrared are usually together but infrared can be present without light. With his 18th experiment, Herschel determined beyond doubt that increasing its power cannot make infrared visible.
Herschel embarked on an extensive instrument-building program to examine and measure each property. To the original prism and mercury thermometers he added a variety of lenses and mirrors in a dozen different configurations and an extensive array of transparent materials to compare transmission.
In more than 200 experiments, he recorded page after page of readings using every available illuminating source viewed through different combinations of mirrors, prisms and lenses. He confirmed again and again, in every way he could test, that light and radiant heat have the same optical properties.
The frantic pace of his later experiments may have caused him to miss or misinterpret connections, especially after he began to look for differences instead of similarities. He did a detailed experiment showing that the focal length of a refractive converging lens was longer for heat than for light, without realizing that the difference in focal length is caused by the same dispersion as a prism.
He measured the effects of scattering, and found, correctly, that light scatters more than infrared. Herschel attributed the difference to light and heat having different natures, rather than as evidence of similar behavior in a different interaction with matter (as scattering is dependent on wavelength).
Herschel did not have a scientist’s insight into the causes of these phenomena, and he had limited ability to formulate mathematical descriptions of his findings. His strengths were his practical knowledge of optics combined with craftsmanship in the fabrication of instruments and his powers of observation.
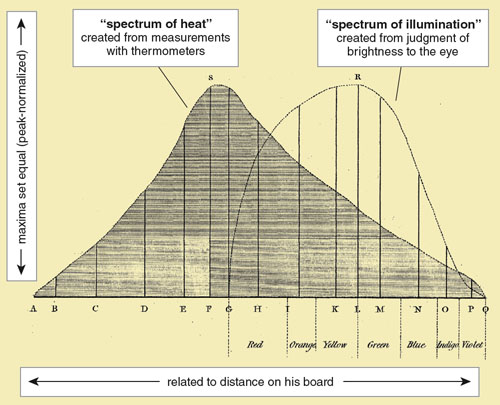
His final paper, presented November 6, 1800, contained the first graph made showing the spectral distributions of visible light and infrared radiation. He called these curves the “spectrum of illumination” and the “spectrum of heat.” On the vertical axis, Herschel plotted measured temperature and perceived brightness. He set their maxima equal (a format called peak-normalization) to compare the relative extent and shape of the distributions and the location of their maxima. For the horizontal axis, not having the concept of wavelength, he used distance in relation to where the visible colors fell. The horizontal axis is reversed from today’s convention of increasing wavelength from left to right.
Herschel’s graph was the product of imagination, insight and months of painstaking work, but it was fatally misleading. Its appearance was probably the deciding factor in his conclusion that light and radiant heat are fundamentally different after all. As he wrote (with the area “ASQA” encompassing the spectrum of heat and “GRQG” the spectrum of illumination):
A mere inspection of the two figures... will enable us now to see how very differently the prism disperses the heat-making rays, and those which occasion illumination, over the areas ASQA, and GRQG, of our two spectra! These rays neither agree in their mean refrangibility, nor in the situation of their maxima. At R, where we have most light, there is but little heat; and at S, where we have most heat, we find no light at all!
The presentation of data can strongly influence their interpretation. Even today, it is difficult to look at Herschel’s graph without the impression that light and radiant heat are two different types of rays. Herschel’s curves are both accurate, but they are of different, almost unrelated quantities and should not be graphed together. His error was not in his basic data but in his assumption that the curves were comparable.
To evaluate Herschel’s spectra, we need to see how the Sun appeared from the village of Slough at the time of his measurements. Herschel did not record the date and time, but analysis of the curve shape indicates the data likely came from his first experiments, and thus were probably taken sometime in late February or early March. Slough lies at latitude 51.5 degrees north, which would make the solar zenith around 61 degrees (29 degrees above the horizon) at local noon.
If Herschel’s measurements had been made in summer, when the Sun is higher in the sky, the maximum he found would have been closer to the center of the visible spectrum. But in winter, with a longer atmospheric path, the maximum of the spectral irradiance (the incident power density as a function of wavelength) is pushed toward red due to atmospheric scattering of the shorter wavelengths.
A computer atmospheric model was used to calculate the distribution of solar irradiance that illuminated Herschel’s prism, and the curve was normalized for comparison. Herschel was meticulous in recording his temperatures, but although he owned a number of prisms of both crown and flint glass with a variety of angles, he did not record what was used in his first experiments. The curve shown assumes his prism was of crown, which was common in 1800, and had a 60-degree angle.
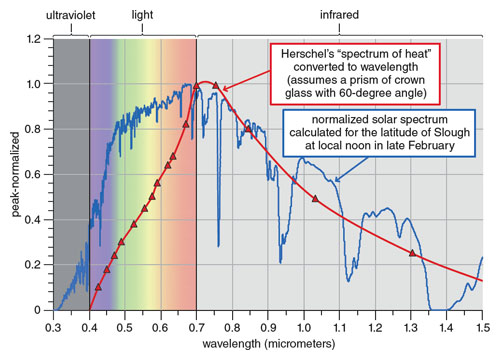
Comparing his spectrum of heat with the solar spectrum shows that Herschel made remarkably good measurements considering the limitations of his instrument and that he had no idea how a solar spectrum should look. His curve is displaced toward longer wavelengths because of the dispersion curve of glass.
Relating the index of refraction to wavelength, the dispersion curve causes refraction to spread the wavelengths across the target board nonuniformly. At each position, moving toward longer wavelengths, the spectral width or band of wavelengths that the thermometer receives becomes progressively wider. A wider spectral width contains more power, which increases the reading. The result gives readings at longer wavelengths that are weighted more heavily than those at shorter wavelengths.
Today, the nonuniform response of a spectrometer can be corrected. But such calibration requires knowledge of wavelength and a source of known radiant power, neither of which were concepts in Herschel’s time. The displacement of his heat curve is not erroneous in itself; it was actually serendipitous because it created the temperature trend that led him into the infrared.
In spite of its displacement, the shape of Herschel’s curve confirms his first hypothesis that the heating power of sunlight is not equally distributed across the spectrum. The fact that his curve is continuous in its transition into the infrared supports his second hypothesis that light and radiant heat are the same quantity.
For the spectrum of illumination, it must have seemed logical to Herschel to plot this on the same graph because his original objective was to find a filter that would maximize light while minimizing heat. His curve was accurate, but it did not show what he thought.
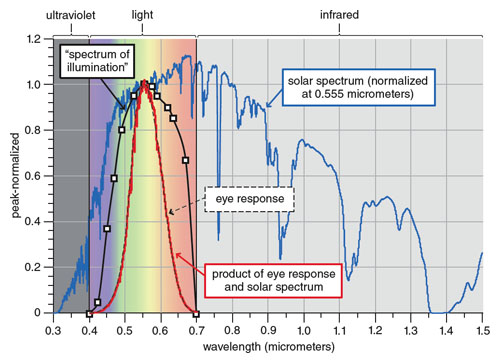
Herschel was missing a concept, unknown in 1800, but fundamental to radiometry today: the effect of sensor response. The reading obtained with any instrument or sensor, including the human eye, results from the product of two curves: the distribution of the received power and the curve of instrument response, which includes the spectral transmission of all optical elements.
If an instrument responds nonuniformly, as most do, then the response shape is impressed on the received radiation in a way that cannot be extricated without independent knowledge of how the radiation is distributed and how the instrument responds. Lacking this concept, Herschel assumed the spectra he measured accurately represented how the radiation was distributed.
Any spectrum created from what the eye sees will be zero outside the eye’s limits. Inside its limits, the power received at any wavelength will be weighted by the eye’s sensitivity to that color. The greatest sensitivity, as Herschel correctly determined, is yellow-green at a wavelength of 0.555 micrometers.
Human color vision is more complicated than shown, but the International Commission on Illumination curve for the light-adapted (or photopic) eye illustrates the concept (see figure 10). The sensation of sight is so rich in information that we don’t often think about how narrow is the slice of the electromagnetic spectrum that we see. Because of its narrowness, the shape of the response curve and that of its product with the solar spectrum are nearly identical.
As a result, Herschel’s spectrum of illumination is more a map of how the human eye responds to colors than how light is actually distributed. He saw the shape of his curve as evidence that light is not equally distributed across the spectrum. His hypothesis was correct, but his data did not show it. He would have gotten an almost identical curve even if the distribution were uniform.
By November 1800, Herschel was approaching the end of what was possible to achieve given the knowledge and technology of his time. He was also almost certainly feeling pressure to return to astronomy.
In the end, the misleading appearance of his graph and the different sensations of seeing light and feeling heat won out over all of his carefully collected evidence, leading him to conclude that the two energies are not the same after all. Actually, he never reached a firm conclusion. The closest Herschel came was to again invoke philosophy, this time to argue against his original thought:
It does not appear that nature is in the habit of using one and the same mechanism with any two of our senses.… Are we then here, on the contrary, to suppose that the same mechanism should be the cause of such different sensations, as the delicate perceptions of vision, and the very grossest of all affections, which are common to the coarsest parts of our bodies, when exposed to heat?
This argument would not be credible today and probably sounded weak even in 1800, but it was a way of bringing his quest to a close. Herschel was surely disappointed, but he had achieved more than he or any contemporary realized.
He discovered that radiant heat has the same optical properties as light. He confirmed his hypothesis that heating power is not equally distributed across the spectrum. He performed the first radiometric measurement of spectral radiant power across the visible into the infrared and found it to be a smooth, continuous curve.
Our present understanding of electromagnetic radiation grew from Herschel’s simple measurements of temperature in sunlight to the unification of the spectrum mathematically by James Clerk Maxwell in 1861 and, ultimately, to Max Planck’s formulation of the quantum theory in 1900. Herschel could not conclusively prove that light and radiant heat are the same quantity, but his experiments provided very strong evidence and were the first piece of the puzzle that others later built on.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.