Hacking Hydrogen
By Dean J. Tantillo
By swapping isotopes, chemists can set up molecular “footraces” that allow them to probe reactions and tune their products.
By swapping isotopes, chemists can set up molecular “footraces” that allow them to probe reactions and tune their products.

In life and in chemistry, not all uphill climbs are created equal. For example, racers come in different sizes; some have built-in advantages that give them a head start. Imagine you are a racer, but your slender cousin always starts 10 meters ahead of you, partway up the hill. She takes off for the summit, and she always surmounts the peak before you do and then rapidly begins her descent. Your race time will always be slower than hers.
Reacting organic molecules can face similar challenges. They must surmount high barriers that slow their passage to products. Many of these reactions involve breaking carbonhydrogen bonds. In such cases, mass makes a difference. Breaking a carbon-hydrogen bond that has the lightest, most common hydrogen isotope will always be faster than breaking an analogous bond between carbon and hydrogen’s heavier cousin, the isotope known as deuterium. The disparity comes from the reaction’s starting point, and the difference in reaction rate is known as a kinetic isotope effect.

Barbara Aulicino/Pexels.com
This unfair advantage isn’t bad news. The kinetic isotope effect is a tool that chemists can exploit to understand how reactions work, to alter the course of reactions, and to change how drugs react within the human body.
Chemists can use kinetic isotope effects to hack into chemical reactions. By strategically substituting hydrogen atoms with heavier deuterium in a key location within a reacting molecule, they can set up a molecular “footrace” and measure whether hydrogen gets a head start. If replacing hydrogen with deuterium slows a reaction, then they know that bond between carbon and either hydrogen or deuterium is likely broken in (or before) the rate-determining transition state. The transition state sits at the summit of the tallest hill and lasts for mere femtoseconds. If the race between hydrogen and deuterium is a tie, the carbon-hydrogen or carbon-deuterium bond likely isn’t broken in that decisive uphill climb.
Because transition-state structures don’t persist, chemists can’t use typical experimental tools to characterize and describe them. Theoretical chemists like me and researchers in my group can help by providing new hypotheses that we generate from computational models. We can calculate kinetic isotope effects from the properties of a transition state and then compare these predicted results to kinetic isotope effects measured from reactions in flasks. If the measured and predicted kinetic isotope effects do not match, our hypothesis is faulty. If the measured and predicted results match, then the computed transition state structure is considered reasonable.
Organic chemists have most often used kinetic isotope experiments to decide between competing proposals for the mechanisms, or paths, of reactions. In many cases, reactants could follow more than one feasible pathway as they convert to products, but which is preferred isn’t always obvious. Discounting apparently viable, but incorrect, mechanisms allows chemists to better understand the details of what’s going on during a reaction, much like police detectives ruling out suspects. With more precise knowledge of when and why bonds break and form, chemists can design new strategies for assembling everything from drugs to plastics.
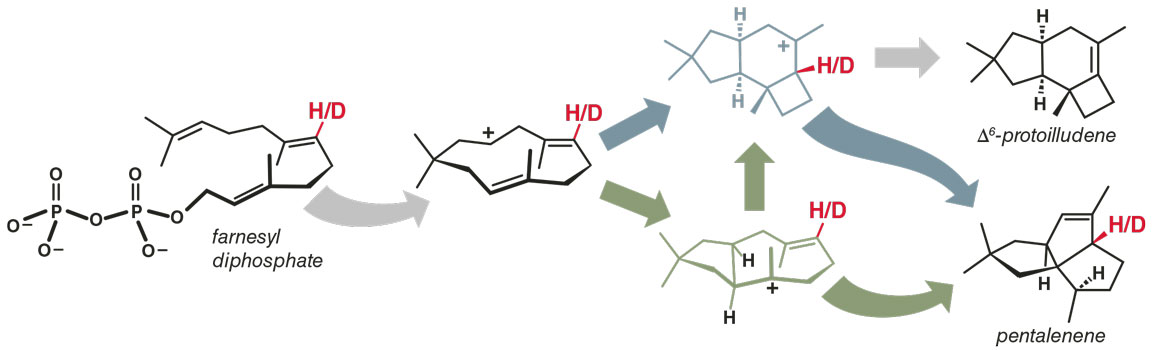
Illustration by Barbara Aulicino.
Our group wanted to test the validity of our theoretical and computational methods for interrogating reaction mechanisms, and we decided to look at the formation of pentalenene from farnesyl diphopshate in the presence of an enzyme called pentalenene synthase (see figure above). The structure was intriguingly complex, and experimental chemists had studied the system, giving us data against which we could compare our theoretical models. In nature, farnesyl diphosphate reacts to produce hundreds of compounds, many of which are converted to compounds with important biological activities, both negative (the aptly named vomitoxin), and positive (the anti-malarial artemisinin). Previously, researchers had proposed a mechanistic sequence to produce pentalenene and another product, ∆6-protoilludene, that included the intermediate shown above in green. However, when my students and I calculated possible intermediates using quantum chemistry, we found the intermediate shown in blue instead, which would mean that the reaction occurred via a different pathway. To decide between the old and the new, we designed a kinetic isotope effect experiment.
Kinetic isotope effects can alter a molecule’s chemistry within living organisms.
For this experiment we used two different versions of farnesyl diphosphate, one with a hydrogen at the position highlighted in red and the other with a deuterium at that position. Our collaborators then carried out the reaction of each molecule with pentalenene synthase and measured the ratio of pentalenene to ∆6-protoilludene produced. If the green model is correct, the product ratios for either version of the starting compound should be approximately identical. If the reaction uses that green intermediate, moving forward along the reaction path does not require breaking the carbon-hydrogen or carbon-deuterium bond immediately.
In contrast, if the blue model is correct, the two reactions should produce very different product ratios. That’s because the pathway that uses the blue intermediate requires breaking a carbon-hydrogen or carbon-deuterium bond right away to form ∆6-protoilludene, but not to proceed forward toward pentalenene. Therefore, the reaction of the deuterated substrate should form the ∆6-protoilludene product more slowly, allowing more of the other product, pentalenene, to form.
When we did the kinetic isotope effect experiment, we saw a large change in the product ratio, evidence that supports the pathway in which the blue intermediate is formed. The previous mechanistic hypothesis, involving the green intermediate, was quashed.
Kinetic isotope effects can do more than serve as tools for decoding how reactions work. They can also direct their outcome. Chemists continue to synthesize complex naturally occurring organic molecules, both to develop new chemical reactions and to find new ways of producing useful amounts of rare molecules with important biological functions, such as antibiotic or anticancer activity. Molecules called welwitindolinones, isolated from blue-green algae, have been popular targets for total chemical synthesis, in part because their molecular architectures are as complex as their tongue-twisting names.
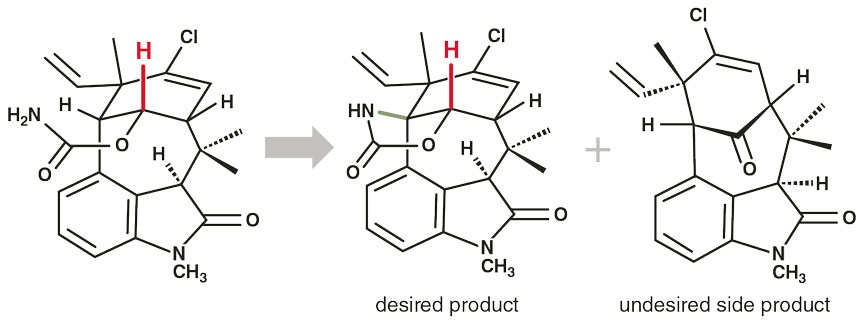
Illustration by Barbara Aulicino.
Neil Garg and his coworkers at the University of California, Los Angeles had devised a complex strategy for synthesizing welwitindolinones, but they faced a vexing synthetic problem. To complete these structures’ skeleton, they needed to form a new carbon-nitrogen bond, which adds a new, critical ring. Unfortunately, this reaction produced both the desired product and an undesired side product in comparable yields (see figure above).
But Garg’s team also noticed important differences in the ways in which the two products formed. The undesired product is produced in a reaction that breaks a carbon-hydrogen bond, but that bond is not broken during the desired reaction. They realized that substituting a deuterium for that critical hydrogen could help steer their synthesis. As a result, they slowed down the unwanted reaction and selectively reduced the yield of the undesired side product.
Kinetic isotope effects don’t just affect chemical reactions going on within laboratory flasks. They can also alter a molecule’s chemistry within living organisms. The human body metabolizes drugs and other foreign molecules: Our cells perform chemical reactions that modify their structures and mark them for removal. Drug developers can use kinetic isotope effects in their designs, both to optimize the potency of an active drug and to tune its lifetime within the body.
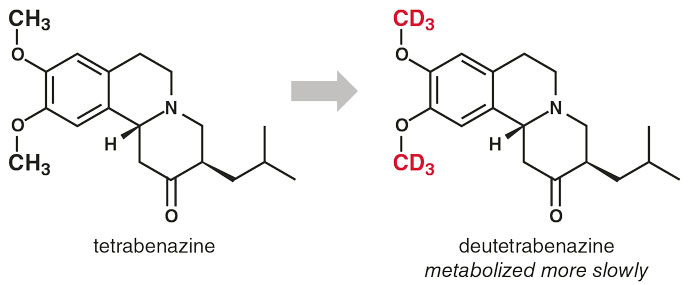
Illustration by Barbara Aulicino.
Doctors can use the drug tetrabenazine to treat hyperkinetic movement disorders, such as those associated with Huntington’s disease, Tourette syndrome, and tardive dyskinesia. One common chemical process in drug metabolism is demethylation, in which enzymes lop off methyl (CH3) groups attached to oxygen or nitrogen atoms. To get the process started, enzymes typically break one of the methyl group’s carbon-hydrogen bonds. Replacing the hydrogen atoms of a methyl group with deuterium atoms should slow down this chemistry and extend the lifetime of the drug in the body (see figure above). Such chemical tweaks can have significant consequences for drug development. A hydrogen-deuterium substitution is the smallest possible chemical change and is therefore unlikely to produce unwanted side effects.
In 2017, the U.S. Food and Drug Administration approved a deuterated drug for the first time: deutetrabenazine, in which all six hydrogens of the two methyl groups on oxygen atoms are replaced by deuterium to slow demethylation. This feature doubles the active species’ lifetime within the body.
The reaction-rate differences that result from breaking carbon-hydrogen bonds rather than carbon-deuterium bonds—the so-called primary kinetic isotope effects—are the most commonly studied and exploited kinetic isotope effects. But they aren’t the only way that heavier isotopes can change the race up reaction hills. Heavier isotopes on bonds neighboring those that break (called secondary kinetic isotope effects) can tweak reaction rates to a lesser extent. In addition, isotopes of carbon, oxygen, nitrogen, and other elements can also produce kinetic isotope effects.
Any change in mass, even a small one, affects the outcome of a reaction race. And those tweaks are often detectable and may be useful in venues ranging from flasks to human interiors.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.