The Rise of Parkinson's Disease
By E. Ray Dorsey, Todd Sherer, Michael S. Okun, Bastiaan R. Bloem
Many cases of this increasingly common neurodegenerative disease are preventable, and we may now know how.
Many cases of this increasingly common neurodegenerative disease are preventable, and we may now know how.

Neurological disorders are the world’s leading cause of disability. And the fastest growing of these conditions is not Alzheimer’s but Parkinson’s disease.
From 1990 to 2015, the number of people living with Parkinson’s more than doubled from 2.6 million to 6.3 million, according to a 2015 study in Lancet Neurology. By 2040, the number is projected to double again to at least 12.9 million, a stunning rise (see figure below).
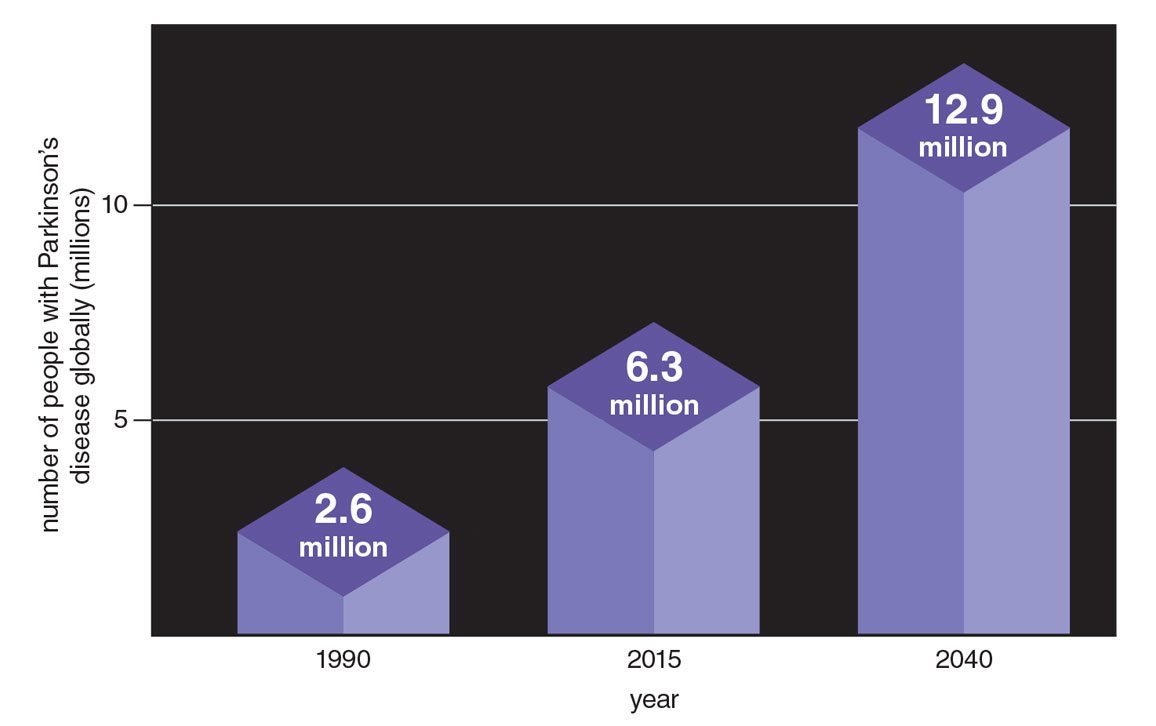
Figure adapted from E. R. Dorsey and B. R. Bloem, 2018.
Neither our increased awareness of the disease nor our lengthening life spans can fully account for the upsurge in diagnoses that we now face. Our knowledge of another neurological disorder, multiple sclerosis, has increased too, and we have improved diagnostic tools for it. Rates for multiple sclerosis have indeed gone up, but that increase is nothing like the exponential rise of Parkinson’s (see figure below). As for aging, more people are, of course, living longer. For example, from 1900 to 2014, the number of individuals over age 65 in the United Kingdom increased about sixfold. However, over that same period, the number of deaths due to Parkinson’s disease increased almost three times faster.
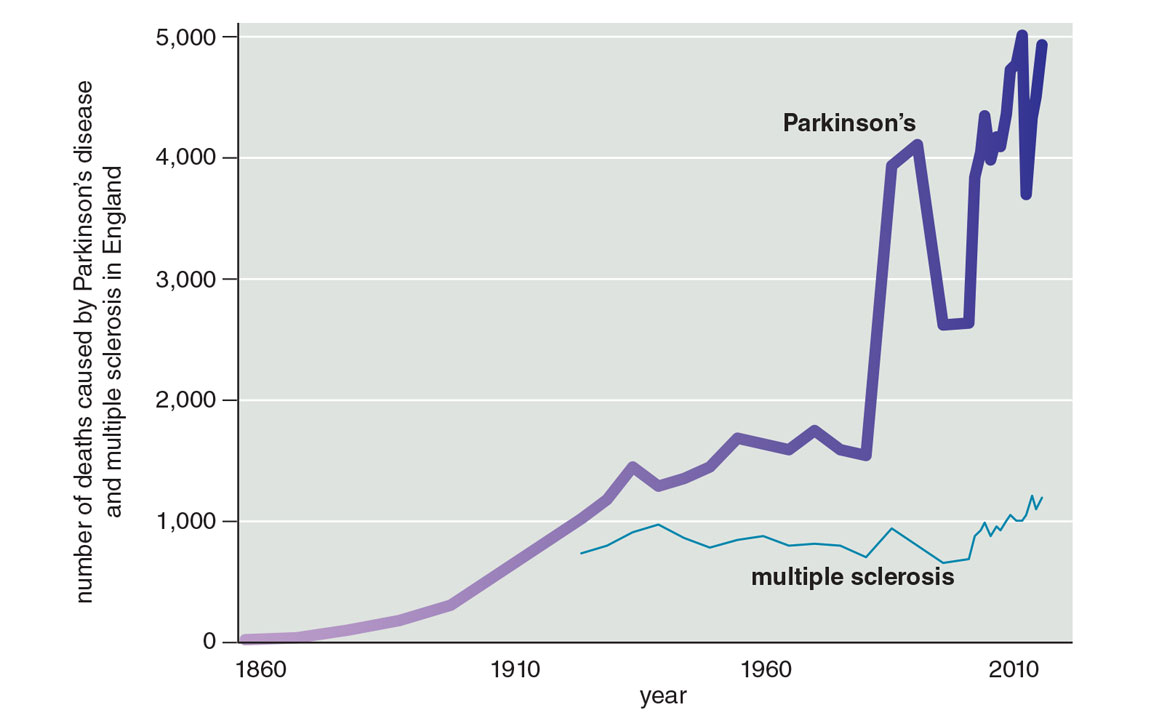
Figure adapted from R. Dorsey et al., 2020.
Parkinson’s disease is characterized by tremors, slowness in movement, stiffness, and difficulties with balance and walking. It can also cause a wide range of symptoms that are not visible—loss of smell, constipation, sleep disorders, and depression. Most people with Parkinson’s are diagnosed in their fifties or later. But it is not just a disease of the elderly. Up to 10 percent of those with the condition develop the disease in their forties or younger.
Parkinson’s stems from a loss of nerve cells in a particular region of the brain that produces dopamine, the brain chemical that helps control movements such as walking. The disease has multiple causes including environmental hazards—air pollution, some industrial solvents, and particular pesticides. In addition, certain genetic mutations, head trauma, and the lack of regular exercise all increase risk.
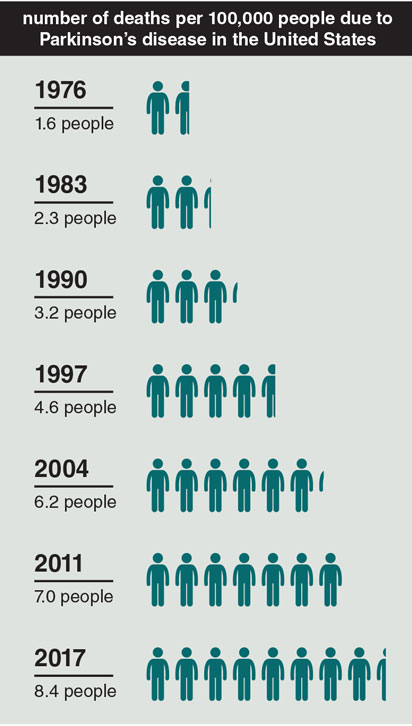
Data from: U.S. National Center for Health Statistics, National Vital Statistics System, Mortality Data.
While industrialization has increased incomes and life expectancies around the world, its products and by-products are also likely increasing the rates of Parkinson’s. Air pollution began to worsen in England in the 1700s, metal production and its harmful fumes increased in the 1800s, the use of industrial chemicals rose in the 1920s, and synthetic pesticides—many of which are nerve toxins—were introduced in the 1940s. All are linked to Parkinson’s—people with the most exposure have higher rates of the disease than the general population.
The evidence for this connection is overwhelming. Countries that have experienced the least industrialization have the lowest rates of the disease, whereas those that are undergoing the most rapid transformation, such as China, have the highest rates of increase. Specific metals, pesticides, and other chemicals have all been tied to Parkinson’s in numerous human studies. When animals are exposed to many of these substances in lab experiments, they develop the typical characteristics of the disease.
Agricultural areas have the highest rates of Parkinson’s. In Nebraska, the rates of the disease are two to four times higher in the state’s rural, agricultural parts than in urban Omaha, according to a 2004 study in Movement Disorders. In Canada, investigators have found an almost perfect correlation between areas with the highest pesticide use and the highest rates of disease, as documented in a 1987 study in the Canadian Journal of Neurological Sciences. In France, rural areas have the highest rates of Parkinson’s, as do the regions with the most vineyards, which often require intense pesticide use, according to a 2017 study in the European Journal of Epidemiology.
Farmers who are exposed to certain pesticides have a higher risk of developing the disease. In one 1998 study published in Neurology, the risk of developing the disease for farmers was 170 percent greater than that for nonfarmers. And the longer farmers have worked with pesticides, the greater their risk.
Pesticide risks are not limited to farmers. People who simply live in rural areas have high rates of Parkinson’s disease. These individuals may be exposed to pesticides in the air that can drift into residential communities. In addition, pesticides can contaminate groundwater or well water. Private wells are often shallow and may be especially at risk for contamination from nearby pesticides. Moreover, in the United States, private well water is not subject to the same regulations as water that comes from public systems.

Christophe Vander Eecken / Reporters / Science Source
And then there are the rest of us. We eat fruits, vegetables, nuts, and grains every day that have been doused in pesticides. What kind of risk are we all exposed to? We do not know. Assessing pesticide exposure from foods and documenting individuals’ dietary habits over a lifetime—given that Parkinson’s takes decades to unfold—are challenging. But this research is needed. Until then, we are left with educated guesses. We do know that organic foods have much lower levels of pesticide residues than conventional choices.
Despite the vast evidence, we are doing little to manage these threats (see “How Property Rights Can Fight Pollution,” March–April 2020). The U.S. Environmental Protection Agency (EPA) had at one time proposed banning one of the chemicals that is tied to Parkinson’s, a solvent called trichloroethylene. But after lobbying by the chemical industry, the EPA decided in 2017 to postpone the ban indefinitely. The uses of trichloroethylene have been so numerous and widespread—in washing away grease, cleaning silicon wafers, removing spots in dry cleaning, and even, until the 1970s, decaffeinating coffee—that almost all of us have been exposed to it at some point in our lives. Some of these uses continue today. Almost half of Superfund sites—land so polluted that the EPA or the responsible parties have to clean it up—are contaminated with trichloroethylene. Thousands of other sites are polluted across the country.
As a result, as reported by the EPA, up to 30 percent of the U.S. drinking water supply has been contaminated with trichloroethylene. Because it readily evaporates from groundwater and soil, the solvent, like radon, can enter homes or offices through the air, undetected. Parkinson’s is not even the most concerning safety risk. According to the EPA, trichloroethylene also causes cancer.
But trichloroethylene is only one dangerous chemical that we have failed to protect ourselves against. Paraquat is a pesticide so toxic that 32 countries, including China, have banned it. Exposure to the chemical increases the risk of Parkinson’s by 150 percent, according to a 2011 study in Environmental Health Perspectives. Yet the EPA has done little. And as the agency charged with protecting our environment sits, paraquat’s use on U.S. agricultural fields has doubled over the past decade, according to data from the U.S. Geological Survey’s Pesticide National Synthesis Project.
The nerve toxin chlorpyrifos is the most widely used insecticide in the country, drenching golf courses and dozens of crops, including almonds, cotton, grapes, oranges, and apples. It has been linked not only to Parkinson’s but also to problems with brain development in children. Again, the EPA has shelved a ban. When a federal court stepped in to take action against the chemical, the Trump administration appealed. And in July 2019, in response to a court ordering a final ruling, the EPA decided that it would allow continued use of chlorpyrifos.
The number of cases of Parkinson’s disease is rising at an alarming rate, but we know how to slow this increase—if we are just able to pay attention to what the epidemiological evidence is telling us. All the evidence indicates that the full effect of the Parkinson’s pandemic is not inevitable but, to a large extent, preventable. The four of us—one neuroscientist and three neurologists who specialize in Parkinson’s—have devoted most of our professional lives to this disease. While we are hopeful about making our patients’ lives better, our true passion is preventing people from ever having to face Parkinson’s. We are frustrated when we see women and men in our clinics who have suffered head trauma or been exposed to pesticides on a farm, solvents at work, contaminated groundwater in their neighborhoods, or polluted air in their homes. All these risks for Parkinson’s can be mitigated. We humans have helped create this pestilence. And we can now work to end it.
Over the past half century, we have begun to identify the worst risks and address them. The insecticide DDT (short for dichlorodiphenyltrichloroethane) was once considered a miracle compound. In the 1930s, the Swiss chemist Paul Hermann Müller was looking for a chemical that could kill insects that were destroying crops and spreading disease—without harming the plants. Müller, a nature lover, tested hundreds of chemicals before coating the inside of a glass box with DDT, a colorless, tasteless, and almost odorless nerve toxin. He placed houseflies into the container, and they bit the dust. Müller had found his answer.

Pictorial Press Ltd/Alamy Stock Photo
In 1948, Müller received the Nobel Prize in Medicine. But DDT was not the miracle chemical that Müller had envisioned. Beginning as early as the 1940s, its harmful effects on the health of wildlife, humans, and the environment were identified and detailed, including in Rachel Carson’s 1962 book Silent Spring. Even though it was banned half a century ago, DDT persists in the environment—and in our food supply. It becomes more concentrated as it makes its way up the chain to human consumption. The pesticide is then stored in our fatty tissues.
We eat fruits, vegetables, nuts, and grains every day that have been doused in pesticides. What kind of risk are we all exposed to? We do not know.
Because of the widespread use of DDT and related chemicals, some are detectable in nearly everyone. In 2003 and 2004, more than 30 years after the insecticide was banned, the U.S. Centers for Disease Control and Prevention (CDC) tested the blood of about 2,000 people ages 12 and older. The researchers were looking for DDT and its metabolite, or breakdown product, dichlorodiphenyldichloroethylene (DDE). They found in their 2009 report that “a small proportion of the population had measurable DDT [and] most of the [U.S.] population had detectable DDE” in their blood. For Parkinson’s, what matters more are the concentrations of chemicals in the brain, which may be several times those in blood, according to the Extension Toxicology Network, because DDT dissolves in fat.
Around the same time that the risks of DDT were being recognized, a newer pesticide was also coming onto the scene. Vietnam veterans and up to 4 million Vietnamese came into contact with Agent Orange during the Vietnam War. Named for the color of the 208-liter barrels in which it was stored, Agent Orange was an herbicide mixture designed to kill vegetation and crops in the tropical forests so that aircraft fighters could spot the enemy below.

Everett Collection Inc./Alamy Stock Photo
From 1965 to 1970, an estimated 45 million liters of Agent Orange were sprayed in Vietnam, according to a 2003 Nature paper. There has been no large-scale study of the effect of this exposure on the health of the Vietnamese or war veterans. Smaller studies, however, have linked Agent Orange to many problems in these populations, including birth defects, cancer, and Parkinson’s, as summarized in a 2007 paper in Science by Richard Stone of the Veterans Health Administration. The evidence is sufficient that veterans who were exposed to Agent Orange and now have Parkinson’s are eligible for disability compensation and health care from the U.S. Department of Veterans Affairs.
Another pesticide adds to the story and is also linked to Parkinson’s. In the 1960s, the U.S. Food and Drug Administration had set a zero tolerance level for a pesticide called heptachlor in foods. In 1978, the Pineapple Growers Association of Hawaii and the state of Hawaiʻi were granted an exemption that allowed heptachlor to be sprayed on pineapples but prohibited their use as livestock feed within one year of spraying the fruit with the chemical. Then, in 1982, it was discovered that some contaminated green pineapple tops apparently had slipped into the feed before the year was up. Testing found that 7 of the 19 dairies on Oʻahu had heptachlor levels in their milk that were three to six times the acceptable state level. Two months after the contamination was detected, Hawaiʻi’s state health director ordered all fresh milk removed from stores and schools.
During those two months, Hawaiians were exposed to high levels of the pesticide. Leland Parks, a scientist at the Pacific Biomedical Research Center in Honolulu, tested samples of breast milk from nursing mothers and found that the average level of heptachlor contamination had increased fourfold.
By coincidence, Japanese researchers had launched a Honolulu Heart Program in the 1960s to follow more than 8,000 men of Japanese ancestry living on Oʻahu for the development of heart disease. As part of that study, individuals completed diet surveys including questions on milk consumption. A subset had agreed to have autopsies performed at the time of their death. The research team examined the brains of 449 of them.
The investigators’ findings, published in 2016 in Neurology, were remarkable. They found that the density of nerve cells in the substantia nigra, the area of the brain affected by Parkinson’s, was lowest in those who consumed the most milk. Researchers also found another potential clue: The brains of those with Parkinson’s were more likely to have the remains of heptachlor. These findings were supported by other studies. According to the CDC, some have found that levels of a related pesticide called dieldrin—which was widely sprayed on corn and cotton in the United States until 1970 (all uses were banned in 1987)—are also more likely to be found in the brains of people with Parkinson’s. The blood levels of these pesticides that dissolve in fat, such as DDT, heptachlor, and dieldrin, are also higher in individuals with Parkinson’s.
Although DDT, heptachlor, and dieldrin were banned from use on crops in the United States and other industrialized countries in the late 20th century, use of the pesticides shifted to less industrialized nations, including India and China. Even though China, for example, has now banned these pesticides, it used to be a major producer and consumer of them. As a result, the Chinese have high concentrations of the residues in milk from nursing mothers and increasing rates of Parkinson’s, according to a 2005 study in Chemosphere.
The effects may be felt for years to come, especially in middle- and low-income nations. Remnants of the chemicals are still found in the milk supply of many countries, including Brazil, China, Ethiopia, and Uganda.
The accumulation of these pesticides in the body is not limited to adults who were exposed. They can be passed on to subsequent generations. As the Hawaiʻi case showed, nursing women who ingest these chemicals from dairy or meat products can deliver them to their babies through their breast milk. DDT and similar pesticides were found in the breast milk of women living in Spain, Nicaragua, Taiwan, and the Spanish Canary Islands as recently as 2014.
This class of pesticides may also cross the placenta into the developing fetus. For example, dieldrin accumulates in body fats and is detectable in the brains of fetuses, according to 2001 research in Environmental Health Perspectives. The long-term effects of DDT, heptachlor, and dieldrin on fetuses and breastfed children—and whether these chemicals can increase risk of developing Parkinson’s—are unknown but concerning.
The United States banned DDT, Agent Orange, and heptachlor in the 1970s and 1980s. However, the United States has not banned all pesticides linked to Parkinson’s. The one with perhaps the strongest link to the disease is still in widespread use: paraquat.
The United States has not banned all pesticides linked to Parkinson’s. The one with perhaps the strongest link to the disease is still in widespread use: paraquat.
Paraquat has been used as a pesticide since the 1950s and is marketed as an alternative to the world’s most popular weed killer, glyphosate, more commonly known as Roundup. Paraquat takes care of weeds that not even Roundup can kill. Today, it is used on farm fields across the United States, and its use continues to increase. The pesticide’s primary uses are for corn, soybeans, wheat, cotton, and grapes.
Paraquat may be an excellent weed killer, but its effectiveness comes at an enormous price. A 2009 study in the American Journal of Epidemiology found that exposure to paraquat and another pesticide called maneb within 500 meters of one’s home increased the risk of Parkinson’s disease by a whopping 75 percent. Two years later, another study in Environmental Health Perspectives found that people who used paraquat—most notably farmers—were 2.5 times more likely to have Parkinson’s than those who did not. A scientist at the National Institutes of Health who has investigated paraquat, Freya Kamel, told Danny Hakim of the New York Times in 2016 that the data were “about as persuasive as these things can get.”

Robert Dein
In the laboratory, paraquat reproduces the features of Parkinson’s disease. In a 1999 study in Brain Research, A. I. Brooks of the University of Rochester and colleagues gave paraquat to mice, and their activity decreased. Paraquat also killed dopamine-producing nerve cells in the rodents’ substantia nigras. The greater the amount of paraquat administered, the greater the number of nerve cells lost.
Even beyond Parkinson’s, paraquat has substantial safety concerns. If it touches your eyes, it can damage the cornea and lead to blindness. If you breathe it in, it can cause internal bleeding. If you swallow a teaspoonful, it is fatal.
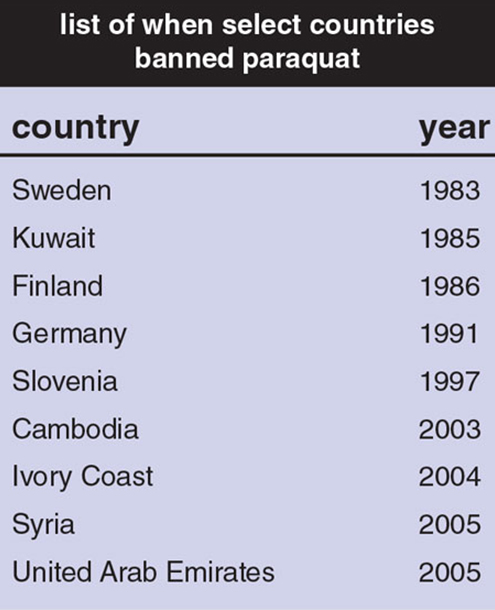
Table adapted from: www.panna.org.
Because of paraquat’s link to Parkinson’s and its role in the deaths of thousands worldwide, 32 countries, including China, have banned its use (see the table on right). However, although the United Kingdom prohibits the use of paraquat, a company there continues to export it to the rest of the world. The company, Syngenta, indicated in an EPA filing quoted in Hakim’s 2016 New York Times report that the evidence of whether paraquat increases the risk of Parkinson’s is “fragmentary and insufficient.” Farmers in Colombia, Ecuador, Guatemala, India, Indonesia, Japan, Mexico, Panama, Singapore, South Africa, Taiwan, and the United States are all customers.
Advocacy groups within and outside the Parkinson’s community have sought to ban paraquat in the United States. On July 24, 2017, the Unified Parkinson’s Advocacy Council, which includes the American Parkinson Disease Association, the Davis Phinney Foundation, the Michael J. Fox Foundation, and the Parkinson’s Foundation, wrote to the EPA, “We write to express our concern with paraquat dichloride, which is shown to increase the risk of Parkinson’s disease. We ask the Environmental Protection Agency to deny reregistration of this herbicide based on strong evidence of paraquat’s harm to human health.”
For pesticides to be sold or distributed in the United States, they have to be registered with the EPA. According to the agency’s own rules, registration is contingent on “scientific studies showing that they can be used without posing unreasonable risks to people or the environment.”
The EPA itself acknowledges the “high toxicity of paraquat” in its online Pesticide Worker Safety publication, “Paraquat Dichloride: One Sip Can Kill.” It includes true stories involving deaths from accidental ingestion of the chemical, including that of an eight-year-old boy who in 2008 drank paraquat that had been put in a Dr. Pepper bottle that he found in the garage. The child died in the hospital 16 days later.
The EPA reviews the safety standards of all herbicides such as paraquat every 15 years. In 2017, they opened paraquat for reconsideration, and it has until October 2022 to make a decision on its future.
In 2019, in the absence of action by the EPA, the Michael J. Fox Foundation submitted a petition with more than 100,000 signatures to the EPA urging it to ban paraquat. Meanwhile, paraquat use in the United States has doubled over the past decade (see the figure below). It is now, according to the EPA, “one of the most widely used herbicides registered in the United States.” In 2016, more than 5.4 million kilograms of it were sprayed in the United States.
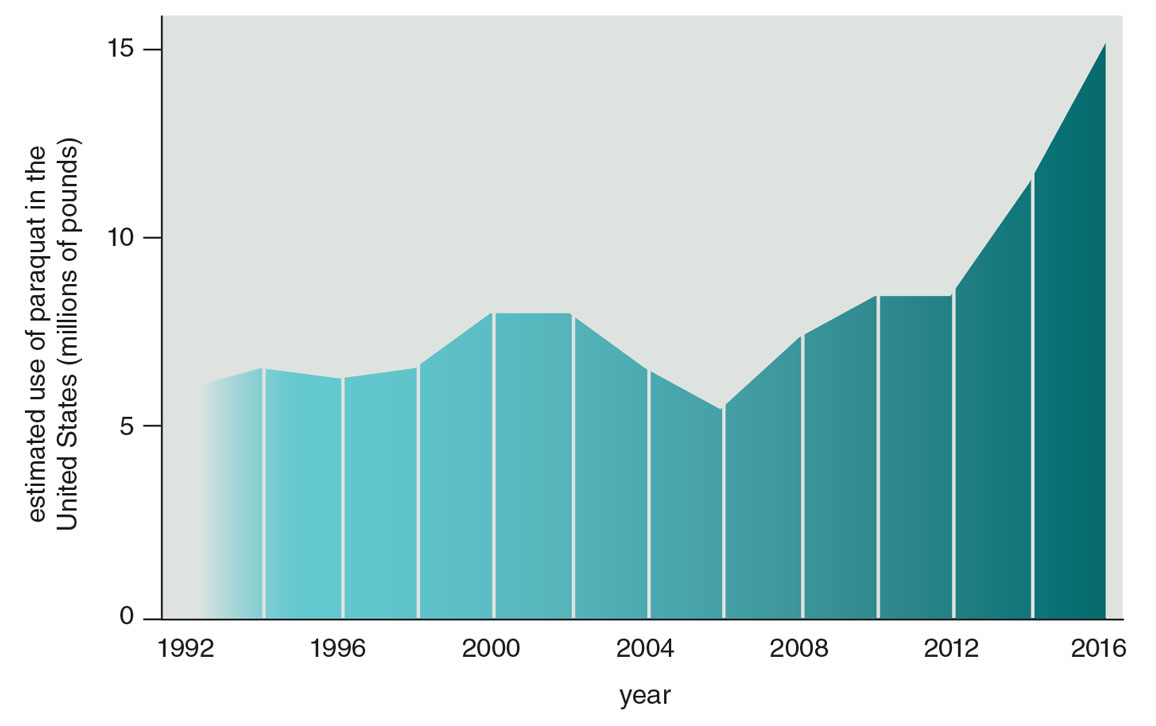
Figure adapted from R. Dorsey et al., 2020.
Appropriate equipment can minimize the harmful health effects of pesticides on humans. For example, protective gloves can reduce the risk of the disease among farmers who are exposed to some (including paraquat), but not all, pesticides. This kind of equipment and additional protective measures, such as boots, disposable coveralls, and goggles, could decrease the risk of developing Parkinson’s disease in the United States and globally.
Challenges remain ahead: There is not only much work still to be done to identify what increases one’s risk of developing Parkinson’s, but also much work to overcome the enormous economic and political pressure that has long favored industry.
In 1964, a decade after demonstrating that smoking causes lung cancer, Sir Austin Bradford Hill gave a lecture to the Royal Society of Medicine that outlined the criteria that an observed association would have to meet to demonstrate causation. The tobacco industry had argued that just because smokers had higher rates of lung cancer was not proof that smoking was the cause. Hill’s achievement was to show that population studies could prove that one led to the other. The link between the two had to be strong, it had to be supported by other scientific evidence, and it had to meet other criteria.
Chemical companies have pushed back against the science on Parkinson’s. Following the tobacco industry playbook, according to a 2018 investigation by Hakim and Eric Lipton of the New York Times, they have lobbied the EPA not to ban certain pesticides by undermining population studies that show the link to various neurological problems, including Parkinson’s. But pesticides and other environmental factors tick most, if not all, of Hill’s criteria. The evidence includes numerous studies demonstrating a strong link between certain pesticides and Parkinson’s, high rates of the disease among those with the greatest exposure, and animal studies that replicate the features of the disease in exposed mice. Based on his criteria, we can conclude that certain pesticides are not merely associated with Parkinson’s. They likely cause it.
Not surprisingly, this conclusion is not shared by those who sell pesticides. A 2016 PLOS ONE review funded by Syngenta reached a different conclusion: “There may be risk factors associated with rural living, farming, pesticide use, or well-water consumption that are causally related to [Parkinson’s disease],” the review says, “but the studies to date have not identified such factors.”
The solution to helping end Parkinson’s disease is not to expose more people to chemicals that are linked to the disease. The solution is to stop using such chemicals. Other countries with lower incomes and less strict environmental policies have banned paraquat (see the table above). The United States should do the same.
Many degenerative diseases reflect the environment that our parents and grandparents created. Just as environmental contamination can worsen health and create disease, cleaning up the contamination can have the reverse effect. Enlightened by science, we now can rectify any prior shortcomings for ourselves and our descendants.
This feature is an adaptation from the authors’ recent book, Ending Parkinson’s Disease. Website: www.endingPD.org.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.