
This Article From Issue
September-October 2004
Volume 92, Number 5
Page 401
DOI: 10.1511/2004.49.401
Along the East Coast of the United States, a cohort of periodical cicadas known as Brood X occupies the prime turf from New York City down to Washington, D.C. Brood Xers are the hip, urban cicadas, the inside–the–Beltway cicadas, the media–savvy celebrity cicadas. When they emerge from their underground existence every 17 years, they face predatory flocks of science writers and television crews, hungry for a story.
This year was a Brood X year. Back in May and June, all along the Metroliner corridor, the air was abuzz with cicada calls, echoed and amplified by the attentive journalists. Then, in just a few weeks, it was all over. The cicadas paired off, fell silent, laid eggs and died. The press moved on to the next sensation. Perhaps a few straggler cicadas showed up days or weeks late, but no one was there to notice, and their prospects cannot have been bright. I worry that the same fate may befall an article about cicadas appearing weeks after the great emergence, at the very moment when most of us want to hear not another word about red–eyed sap–sucking insects for at least 17 years. I beg my readers' indulgence for one long, last, lonely stridulation as the summer comes to a close.
Periodical cicadas are remarkable in many ways, but I want to focus on one of the simplest aspects of their life cycle: the mere fact that these insects can count as high as 17. Some of them count to 13 instead—and it has not escaped notice that both of these numbers are primes. Of course no one believes that a cicada forms a mental representation of the number 17 or 13, much less that it understands the concept of a prime; but evidently it has some reliable mechanism for marking the passage of the years and keeping an accurate tally. That's wonder enough.
The physiological details of how cicadas count will have to be worked out by biologists in the lab and the field, but in the meantime computer simulations may help to determine how precise the timekeeping mechanism needs to be. Computer models also offer some hints about which factors in the cicada's ecological circumstances are most important in maintaining its synchronized way of life. On the other hand, the models do nothing to dispel the sense of mystery about these organisms; bugs that count are deeply odd.
A Clockwork Insect
Cicadas spend almost all of their lives underground, as "nymphs" feeding on xylem sucked out of tree roots; they come to the surface only to mate. Most species have a life cycle lasting a few years, but individuals are not synchronized; all age groups are present at all times, and each year a fraction of the population emerges to breed. True periodicity, where an entire population moves through the various stages of life in synchrony, is extremely rare. Of 1,500 cicada species worldwide, only a handful in the genus Magicicada are known to be periodical; all of them live in North America east of the Great Plains.
Brian Hayes
The taxonomy of the Magicicada group is somewhat controversial and more than a little confusing. If you sort a collection of specimens by appearance or mating call or molecular markers, they fall into three sets; but it turns out that each of these sets includes both 13–year and 17–year forms. Are there six species, or only three? Complicating matters further, John R. Cooley, David C. Marshall and Chris Simon of the University of Connecticut have recently identified a seventh variety that has a 13–year period but shows genetic affinities to a 17–year group.
Then there is the division into broods. A brood is a synchronized population, in which all individuals are the same age. Generation after generation, they go through life in lockstep. One might expect that a brood would consist of a single species, but that's generally not the case. Brood X, for example, includes the 17–year forms of all three species. Geographically, adjacent broods tend to have sharp boundaries, with little overlap. Where two broods do share the same real estate, they are chronologically isolated, typically with four years between their emergences.
If you are a cicada trying to emerge in synchrony with all your broodmates, there are two problems you need to solve: First you must choose the right year, and then the right day (or night, rather) within that year. The latter task is easier. Cicadas synchronize the night of their emergence by waiting for an external cue: They crawl out of their burrows when the soil warms to a certain temperature, about 64 degrees Fahrenheit.
Keeping track of the years is more challenging. First you need an oscillator of some kind—a device that goes tick–tick–tick at a steady pace. The cicada oscillator presumably ticks once per year. Second, you need to tally the successive ticks, like a prisoner scratching marks on the wall of a cell. Finally you have to recognize when the tick count has reached the target value of 13 or 17.
The cicada oscillator is probably an annual variation in some property of the xylem the insects consume, reflecting a deciduous tree's yearly cycle of growing and shedding leaves. Support for this hypothesis comes from an ingenious experiment conducted by Richard Karban, Carrie A. Black and Steven A. Weinbaum of the University of California, Davis. They reared cicadas on orchard trees that can be forced to go through two foliage cycles in a single year. Most of the cicadas matured after 17 of the artificially induced cycles, regardless of calendar time.
The cicada's tally mechanism remains unknown. One example of a biological counting device is the telomere, a distinctive segment of DNA found near the tips of chromosomes in eukaryotic cells. Each time a cell divides, a bit of the telomere is snipped off; when there's none left, the cell ceases to replicate. Thus the telomere counts generations and brings the cell line to an end after a predetermined number of divisions. Perhaps the cicada employs some conceptually similar countdown mechanism, although the biochemical details are surely different.
There is no reason to suppose that cicadas count strictly by ones. Indeed, the coexistence of 13–year and 17–year periods suggests other possibilities. For example, the two life cycles might be broken down as (3x4)+1=13 and (4x4)+1=17. In other words, there might be a four–year subcycle, which could be repeated either three or four times, followed by a single additional year. An appealing idea is to identify such subcycles with the stages, or instars, in the development of the juvenile cicada. And it's notable that what distinguishes 17–year from 13–year forms is a four–year prolongation of the second instar. Unfortunately for the hypothesis, the rest of the nymphal stages are not uniform, four–year subcycles. Nymphs pass through them at different paces. Only at the end of the cycle do the members of a brood get back in synch.
Missing a Beat
The synchronization of cicada emergence is impressive, but not perfect. There are always at least a few clueless unfortunates who turn up a year early or a year late. Four–year accelerations and retardations are also common. Evidently, the year–counting mechanism can go awry. How much error can the system tolerate before synchronization is lost entirely?
Several authors have proposed that Magicicada periodicity evolved during the Pleistocene epoch, as a response to the unfavorable and uncertain climate of glacial intervals. Conditions have changed dramatically since the glaciers retreated, and so it seems unlikely that the same selective pressures are still working to maintain synchronization. What does maintain it? Before considering more complicated hypotheses, it seems worthwhile to ask whether periodicity could have survived as a mere vestigial carryover, without any particular adaptive value in the current environment. If the timekeeping device is never reset, how accurately would it have to work to maintain synchronization over the 10,000 years or so since the end of the Pleistocene?
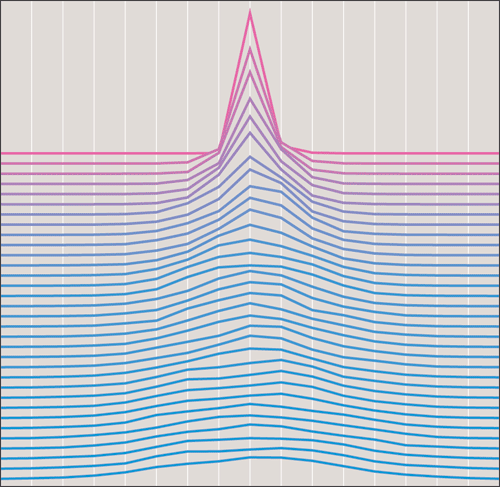
The answer depends in part on what kinds of errors can disrupt the counting. The simplest model allows individual cicadas to make independent errors. Each year, each cicada has some small likelihood of either failing to note the passage of the year or interpolating a spurious extra year. Under this model, the error rate needs to be kept below 1 in 10,000.
The weakness of this model is the assumption that cicadas would make independent errors. If all the cicadas are trying to read the same chemical signal in the tree sap, errors could be strongly correlated. In a bad year with a short growing season, the signal might never reach the threshold of detection for many individuals. A double oscillation is also a possibility, for example if the trees are defoliated by predators and then put out a second growth of leaves.
An error model that allows for such correlations works like this: A cicada's probability of correctly recording the passage of a year depends on the strength of the xylem signal, which varies randomly from year to year but is the same for all the cicadas. If the signal is very strong, almost everyone detects it correctly. If the signal is extremely feeble, nearly all miss it. Although this latter event must be counted as a timekeeping error, it does not break synchronization; instead it retards the entire population by a year. What spoils synchronization is an ambiguous signal, one in the gray area where half the cicadas detect it and the other half don't. This splits the population into two groups, which will mature and emerge a year apart. Four or five such splittings over 10,000 years would be enough to wipe out synchronization.
A drawback of this error model is that it depends on two variables, which are hard to disentangle: the frequency of ambiguous signals in the xylem and the cicada's acuity in reading those signals. If the signal is usually near the extremes of its range, then even with a crude detector, the population will almost always reach a consensus. If ambiguity is common, then the insect's decision mechanism needs to be finely tuned. I have experimented with tree–ring data as a proxy for the distribution of xylem–signal amplitudes, but the results were not much different from those with a random distribution.
The cicadas' response to the signals is defined by an S–shaped curve. If the curve is infinitely steep—a step function—then the probability of registering a tick of the clock is exactly 0 up to some threshold and exactly 1 above the threshold. As the curve softens, the transitional region where probabilities are close to ½ gets broader.
Running the simulation, it turns out that synchronization survives only if the response curve is very steep indeed, with a vanishingly narrow region of ambiguity. For ease of analysis, suppose we are merely trying to synchronize the clocks of two cicadas that each live for 10,000 years. To a first approximation, they remain in phase only if they agree on the interpretation of the signal every year throughout the 10,000–year interval. For a 90–percent chance of such uninterrupted agreement, the probability of agreement each year must be at least 0.99999.
Is such accuracy plausible in a biological mechanism? Could periodicity really be a historical relic, without adaptive significance today? Probably not, but the models are too simplistic to support quantitative conclusions. Nevertheless, the idea of timekeeping errors introduced by ambiguities in environmental signals may well have a place in the biology of cicadas. Suppose there is a north–south gradient in signal amplitude; then somewhere along the gradient there must be a zone of ambiguity. Forty years ago, Richard D. Alexander and Thomas E. Moore of the University of Michigan, Ann Arbor, pointed out that broods tend to be arranged like shingles from north to south, with each brood emerging one year later than the one above. It's a pattern that might have been generated by successive population–splitting events like those in the model.
No Thank You, Not Another Bite
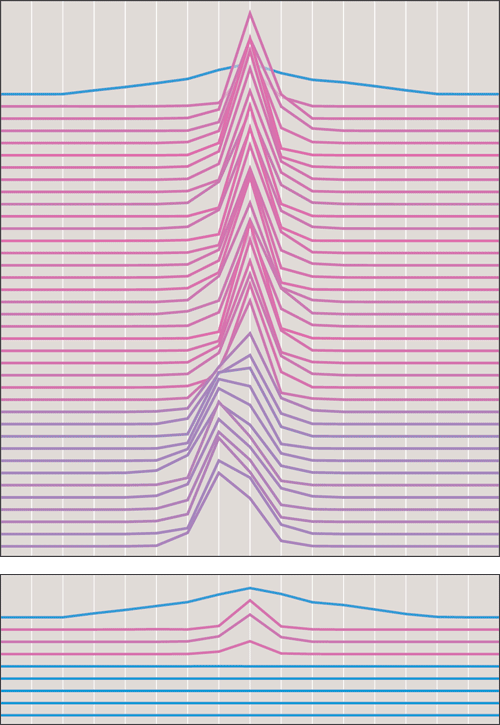
If periodicity confers some current selective advantage, a widely favored candidate for that benefit is "predator satiation." Cicadas are large, noisy, clumsy and tasty (or so I'm told). All in all, they are a bonanza for birds and other predators. But the emerging adults appear in such enormous numbers—often hundreds per square meter—that the feast is more than the diners can finish. If the same mass of cicadas were spread out in yearly cohorts one–seventeenth as large, predators could gobble them up, year by year.
Brian Hayes
Predator satiation and timekeeping errors have the potential to interact in interesting ways. By eliminating off–year stragglers, predation tends to sharpen the peak of the emergence, but of course it also diminishes the height of the peak itself. If predation is too light, then error suppression will be ineffective and synchronization will fail. If predation is too severe, the whole population risks extinction. This analysis suggests the possibility of a system stable only within a narrow range of parameters—always a cause of skepticism, since the parameters are unlikely to be so well–behaved in nature. To my surprise, however, a simple model of predator satiation proved to be highly robust. I defined the satiation threshold—the maximum number of cicadas eaten by predators—as a fraction of the total carrying capacity, or in other words the maximum possible cicada population. I found that synchronization was maintained even with a satiation threshold as low as 0.1; at the other end of the scale, predation did not lead to immediate extinction until the threshold was raised above 0.7.
Other aspects of the model are not so confidence–inspiring. Having added a death rate via predation, it is necessary to include a birth rate as well, or else the population would inevitably dwindle away. The numerical value assigned to the birth rate is somewhat arbitrary. The only guidance comes from field studies showing that successful female cicadas lay a few hundred eggs. Fortunately, the behavior of the model is not overly sensitive to choices of birth rate within the plausible range.
A closely related issue is how to impose a limit on cicada numbers when excess births cause the population to exceed the carrying capacity of the environment. In formulating the computer model, I chose to limit total population by reducing the newborn generation as necessary. Hatchlings could survive only if there were vacancies available for them; newborns could never displace older cohorts. This decision was based on an observation by Karban that infant mortality dominates cicada demography; if a cicada survives its first two years, it will likely last to maturity.
Brian Hayes
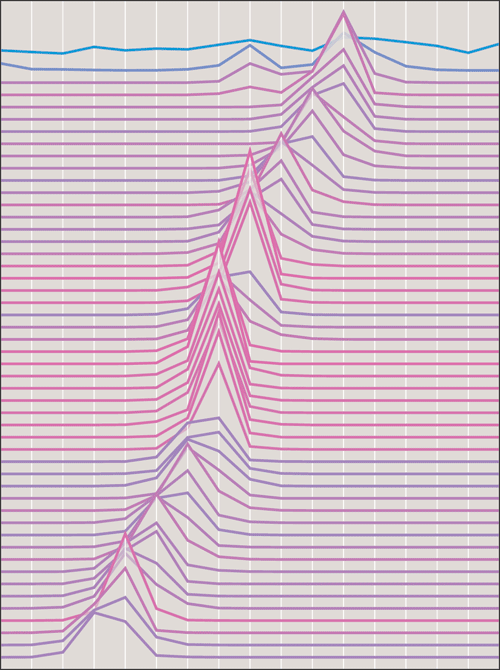
Further experiments revealed that the capacity limit and the strategy chosen for enforcing it can have significant effects on the outcome of a simulation. For example, steady attrition in the nymph population, even at a low background rate of mortality, leaves the brood much more vulnerable to extinction. In contrast, allowing intergenerational competition—in which newborns compete with their elders on an equal basis for the available resources—helps stabilize the synchronized, periodical mode of reproduction. As a matter of fact, it is only in models with this form of competition that I have seen synchronization arise spontaneously from an initially random state. The other models described here can stabilize existing periodicity but have a hard time generating it in the first place.
The crucial role of a finite carrying capacity in cicada population models was pointed out almost 30 years ago by Frank C. Hoppensteadt and Joseph B. Keller, then of the Courant Institute of Mathematical Sciences at New York University. I had read an account of their computer simulations before attempting my own, but only after some direct experience did I understand the emphasis they put on carrying capacity. The importance of intergenerational competition was stressed by M. G. Bulmer of the University of Oxford at about the same time.
A static population near the carrying capacity, low mortality except at the extremes of the age range, reproduction postponed until the last possible moment—these are characteristics of the Magicicada way of life. I can't help noting that the same traits will soon describe the human population. Intergenerational conflict over resources is also conspicuous in human affairs. Perhaps the cycles of baby booms in recent decades are signs of incipient synchronization in human reproductive practices.
Primes and Other Conundrums
One factor not addressed by the models I have described so far is feedback from prey to predator species. In an emergence year, birds that feast on cicadas should be able to raise more young than usual; the resulting increase in predator numbers will make life even harder on straggler cicadas the following year, thus sharpening the population peak. Many authors have drawn attention to this linkage, and have offered it as the key to understanding why the Magicicada species chose prime numbers for their life–cycle periods. Because a prime has no divisors other than itself and 1, the cicadas avoid falling into resonance with predators whose abundance fluctuates on some shorter cycle. For example, a hypothetical 12–year cicada would be susceptible to predators with cycle lengths of 2, 3, 4 or 6 years.
G. F. Webb of Vanderbilt University has constructed computer simulations in which such interactions of prey and predator cycles favor the 13–year and 17–year cicada periods. Eric Goles of the University of Chile and Oliver Schulz and Mario Markus of the Max–Planck Institut für molekulare Physiologie in Dortmund, Germany, have also published on this subject, referring to cicadas as "a biological generator of prime numbers." Whether the cyclic predator species exist remains an open question. And some quite different explanations have also been put forward. For example, Randel Tom Cox of Arkansas State University and C. E. Carlton of Louisiana State University argue that the heart of the matter is not predation but hybridization. Interbreeding between broods that differ in period could disrupt synchronization for both groups. Thus 13–year and 17–year broods are favored because they emerge together only once every 221 years.
Much else about the lives of cicadas remains mysterious. In the 1960s Monte Lloyd of the University of Chicago and Henry S. Dybas of the Field Museum of Natural History in Chicago offered a curious meta–theory of cicada evolution. Any theory that seems too plausible, they argued, is automatically suspect. "If there were a broad, easy evolutionary highway towards periodicity, then why would not more species have taken it?"
If one day we run out of cicada mysteries to solve, there are still harder problems waiting. Synchronized, periodic breeding is known in plants as well as animals. Daniel H. Janzen of the University of Michigan has written about the bamboo Phyllostachys bambusoides, which apparently maintains synchronized flowering even when seeds are planted continents apart. And the length of the plant's period makes the cicadas seem as ephemeral as mayflies. The bamboo knows how to count not just to 17 but to 120.
© Brian Hayes
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.