Aging: A Biological Perspective
By Robert Arking
A variety of techniques extend the lives of model organisms, and similar approaches might help human beings stay healthy longer
A variety of techniques extend the lives of model organisms, and similar approaches might help human beings stay healthy longer

DOI: 10.1511/2003.38.508
People spend more time trying to avoid aging than trying to understand it. We deny aging at first and then—seeing its reality in the mirror—grudgingly accept it. For some reason, the biology of aging attracted my attention early on, during my graduate-student days.
At that time, most other scientists regarded the study of aging as a somewhat misdirected and not quite respectable activity, and so I studied other topics. In the past two decades, though, biological investigations of aging grew into cutting-edge science. Unfortunately, little of that intellectual excitement reaches the larger society.

DiMaggio / Kalish / Corbis
I realized the complicated job of explaining the biology of aging after one conversation with my mother-in-law, who was a pragmatic country woman, well rooted in her time and place. She had apparently made her peace with the fact that one of her sons-in-law made his living by playing around with flies. But in 1979, I told her that I was changing my research focus from studying fly development to studying how to make flies live longer. She looked at me for a long minute with an expression that told me she was reconsidering her daughter's taste in men. Finally, she leaned over and said to me in a very tolerant way, "Bob, we don't want flies to live longer." I had no good reply at the time, but today I could tell her in exhaustive detail that she was partly wrong. We do want flies to live longer, at least in the laboratory, for only then will we be able to make ourselves live longer, too.
As we learn more about aging, we will think more deeply about the consequences of our increasing ability to alter this biological process. To provide an overview of this field, this article defines aging in modern biological terms, describes our current understanding of the biological mechanisms that underlie aging, reviews successful cases of longevity intervention in laboratory animals and discusses the implications for humans. Taken together, this information makes an intriguing tale.
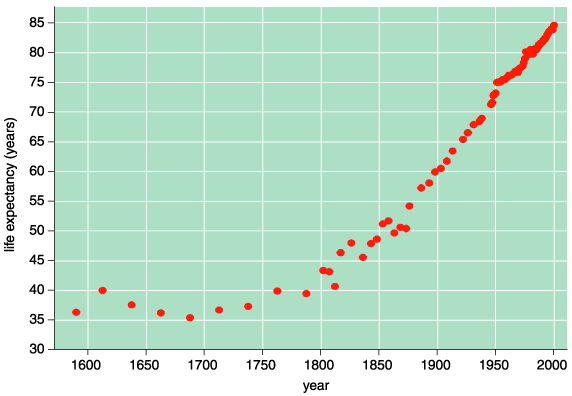
As early as 1840, life expectancy started increasing in Sweden. Soon, the trend appeared in other developed countries, too. In the United States, for example, white females in 1900 lived an average of 48 years; by 2000, they lived an average of 87 years. This 39-year increase in average lifespan really took hold by mid-century, largely due to reduced mortality before puberty, which killed 24 percent of the women born in 1900.
Young girls—no longer taken by accidents or infectious diseases—survived to die as old ladies. In the 1920s, extensive public-health measures, including sanitary sewers and clean drinking water, triggered this decreased mortality. Later, antibiotics and improved medical care increased average life spans even more. As the 20th century rolled by, the elderly grew more healthy and mentally independent than their parents at the same ages. In addition, the average person lived longer than ever. Consequently, the probability of some proportion of them surviving for more than a century increased, as well.
Although social and medical interventions helped people live longer, none of the techniques affected the aging process. A healthy 65-year-old in 1900 would be physically indistinguishable from his or her counterpart in 2000. There are simply more 65-year-olds today because the past century's efforts reduced early mortality. If you do not die young, then you can live to be old, but you will still age as humans have throughout history.
Aging involves multiple deleterious biological events that accumulate in different tissues over time and gradually reduce an organism's state of maintenance and function. Calendar time, however, serves as an imperfect measurement of the physiological processes involved in aging. We all know individuals who are the same chronological age but appear to be very different physiological ages. Rather than counting years—or gray hairs, for that matter—modern gerontologists turn to biological markers, or biomarkers, of aging. These physiological parameters indicate an individual's functional level, and some biomarkers, such as insulin levels, correlate with mortality. The presence of such biomarkers depends indirectly on patterns of gene expression, which are induced by a variety of internal or external stimuli. If gene expression remains stable, so does an adult's overall health. In fact, extraordinary stability in gene expression can create a centenarian, but especially unstable expression can trigger premature mortality. If aging is a series of increasingly different molecular and physiological signatures, then senescence comprises the processes that are responsible for the changes in those signatures.
"Nothing in biology makes sense except in the light of evolution," according to the well-known geneticist Theodosius Dobzhansky, and his statement applies to aging. By natural selection, some genetic variants of any population will be more successful—putting more copies of their genes into the next generation—than others, and the more numerous variants will be favored. Moreover, the high mortality rates resulting from predation, illness and accidents that are common among wild populations indicate that few, if any, individuals live long enough to show signs of aging and senescence. So any wild population consists primarily of young, breeding adults who make the genetic contributions to the next generation. Consequently, deleterious genetic variants that act late in life are not selected against because their carriers probably either die from environmental hazards before they reach old age or survive as post-reproductive adults. In either case, those genes are invisible to natural selection. In addition, long-lived genetic variants will not be selected for because they are expressed only in those few surviving post-reproductive individuals.
From an evolutionary perspective, the entire reproductive game revolves around passing copies of genes to the next generation. No trait, including extended longevity, provides evolutionary value unless it makes an individual more successful in this game. Living long enough to reproduce does merit evolutionary value, but living long enough to be post-reproductive supplies no increased fitness, at least in the case of non-social animals.
Tom Dunne, adapted from Oeppen and Vaupel.
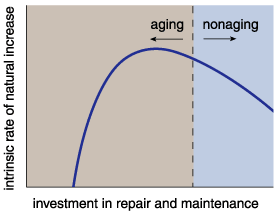
People already live long. Why then are we not capable of reproducing and living indefinitely or at least much longer than we do now? The answer involves energy. An organism must divide its energy between maintenance, repair and reproduction. Even a well-fed organism copes with energy limitations. As a result, organisms face a tough problem: What is the best allocation of finite metabolic energy to maximize reproduction and repair?
In 1977, Thomas Kirkwood of the University of Newcastle Upon Tyne showed theoretically that increasing the amount of energy expended on somatic repair results in increased survivorship but decreased fecundity, and vice versa. A choice must be made. Reproduction requires less energy than does repair. Therefore, allocating sufficient energy to maximize somatic repair will reduce fecundity, which decreases an organism's Darwinian fitness. In contrast, increasing fecundity will decrease the energy available for repair and probably result in a shortened longevity. In most cases, decreased fecundity over a longer life span yields fewer copies of an individual's genes in the next generation than does higher fecundity over a shorter lifetime. Accordingly, maximum fitness takes place at a repair level lower than that required for indefinite somatic repair, and organisms eventually die. This is the so-called disposable soma theory.
This theory reveals an intriguing point: An organism ages when energy allocations fail to make adequate repairs, not because of a genetically based aging program. As a result, humans are not required to age. So if people age only because there is no biological reason not to, then some intervention might stop—or at least slow—aging.
The lives of animals can be lengthened in three ways: increasing their early survival rate, increasing their late survival rate or delaying senescence. The first two approaches decrease the mortality rate at the beginning or end of life, respectively, but do not affect the basic aging process. Even with increased early or late survival, organisms age normally but seem to be somewhat more resistant to stresses that kill off their normal comrades. Humans who exercise, for example, survive at higher rates in early and middle life and experience a lower level of morbidity, or occurrence of disease, but they age normally, with no decrease in late-life mortality. Centenarians, on the other hand, live longer than most people, although no one would mistake a centenarian for a middle-aged person. Instead, centenarians age normally, but tend to be healthier than their ordinary fellows. Although athletes and centenarians provide interesting examples of increasing early and late survival rates, they shed little light on basic aging processes.
Tom Dunne
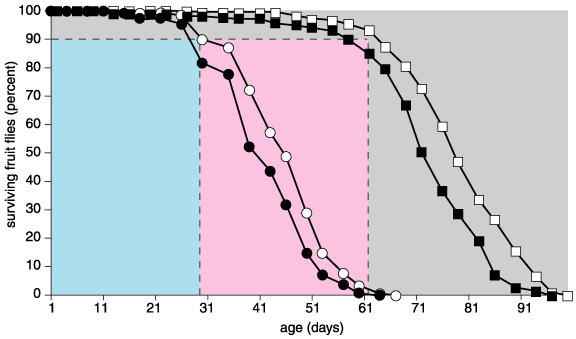
The most interesting alteration involves the third approach, delaying the onset of senescence. Many examples of this pattern exist in laboratory animals, but none in humans as yet. My colleagues and I, for instance, created long-lived strains of fruit flies through artificial selection—simply allowing only the longer-lived flies to breed with one another over several generations. When we compared ordinary and long-lived fruit flies, the average life was about 40 and 70 days, respectively. Likewise, the maximum lifespan increased from about 61 to 91 days. But how much of that added time is lived in good health? Let's call that good-health period the health span and make it the time from birth until ten percent of the initial population dies. For ordinary flies, the health span lasted 30 days, and it grew to 60 days in the long-lived flies. So the flies' health span doubled. Nonetheless, the senescent period—from the end of the health span until all of the flies died—remained the same, about 30 days, which is a smaller proportion of the maximum life span for the long-lived flies.
My lab's data on fruit flies demonstrate that the health span and the senescent span are separate phases of the life span. Moreover, these data reveal that increasing the health span can extend longevity. In fact, experiments from a wide variety of investigators show that delaying senescence can increase longevity in all of aging research's model organisms: common brewer's yeast (Saccharomyces cerevisae), nematode worms (Caenorhabditis elegans), fruit flies (Drosophila melanogaster) and laboratory mice (Mus musculus). For example, Martin Holzenberger and his colleagues at the Institut National de la Santé et de la Recherche Médicale in Paris inactivated the insulin-like growth factor type 1 receptor (IGF-1R) in mice, and the health span of females, (but not males,) increased by about 75 percent. Such findings suggest a built-in potential for increasing the health span, and this potential has been exploited experimentally as described below.
In 1934, Clive McCay and Mary Crowell of Cornell delayed the onset of senescence in rats by reducing how much they ate. In fact, reducing the calories in an animal's diet by about 40 percent, while keeping the different nutrients at normal levels, results in healthy and long-lived mice and rats. It also works for flies and worms. In fact, hundreds of studies show that caloric restriction lengthens life. Similar experiments are underway in macaque monkeys, and, although these long-term experiments are still in progress, the available data suggest that caloric restriction may lengthen primate lives, too. There is good evidence, put forth by Mark Lane and his colleagues at the National Institute on Aging, suggesting that caloric restriction utilizes a hormonal mechanism to exert its effects on all the different tissues of the body.
Tom Dunne
Caloric restriction creates measurable changes in a variety of biomarkers. For example, George Roth and his colleagues at the National Institute on Aging put male rhesus monkeys on a 30-percent reduction in calories for three to five years. When compared with control monkeys, the ones on reduced calories displayed significantly lower body temperature, reduced plasma levels of insulin and increased serum levels of dehydroepiandrosterone sulfate, a steroid hormone precursor molecule that commonly decreases in aging monkeys and humans. We do not yet known if these monkeys will in fact live long, but it is interesting to note that these same three traits were found in long-lived human males.
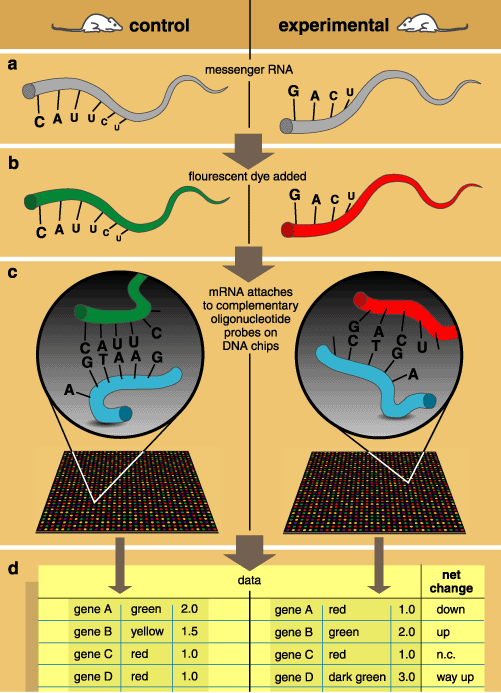
Scientists can also correlate gene-expression patterns with differential mortality for adults of different ages or who received different treatments. This maps physiological function and mortality on the expression states of the genome, which can be determined with DNA chips, or microarrays (see Figure 5). In essence, a matrix of hundreds or thousands of oligonucleotides arranged in a matrix on a slide makes up a DNA chip. Messenger RNA (mRNA) newly transcribed from the genes in an experimental sample or a control can be labeled with fluorescent tags and each then applied to its own chip. If a section of nucleotides on a piece of mRNA being tested complements one of the attached oligonucleotide probes, then the two pieces of chromosomal material hybridize. Non-hybridized material is washed off. Finally, a scanner measures the level of fluorescence at each spot on the matrix, so that the expression of the experimental and control samples can be compared.
Tom Dunne
These microarrays can distinguish between gene-expression patterns of healthy and sick animals or young and old ones. Such microarrays can even differentiate between normal-lived and long-lived animals, despite the difficult statistical problems inherent in the technique. My comparison of several independent experiments leads to the reasonable assumption that each tissue has its own characteristic aging pattern. Skeletal muscle and heart muscle, for example, are very similar to each other, but they age in very different manners. Although both of these are different than neural tissue, certain broad functional similarities exist. All tissues show an increased stress response, likely coupled to increased levels of highly reactive molecules called reactive oxygen species (ROS), or less accurately free radicals, and to an increased level of cellular damage. Aging tissues also seem less capable of processing signal proteins or synthesizing and degrading other proteins. Both of these processes—increased ROS production and decreased signal sensitivity/protein turnover—might set off positive feedback cycles that progressively degrade an aging cell's performance in these and other areas.
Caloric restriction, on the other hand, brings about a variety of changes that seem to have the effect of maintaining the optimal function of the tissue by reducing the stress levels within the cell while retaining the optimal metabolic, biosynthetic and turnover capabilities of the cells. As a result, the animals shift their focus from growth and reproduction to somatic repair and maintenance. The restricted animals age more slowly and maintain their tissue integrity well into old age. They also show either a significant delay or a complete elimination of the onset of many age-related pathologies, and their survival curves are characterized by a significant extension of the health span. In addition to aging more slowly, calorically restricted animals stay physically healthier and mentally active much longer then normally fed controls. On the other hand, calorically restricted animals grow more slowly than normal, are often less fecund and are sometimes less resistant to environmental stresses.
Although reducing calories makes lab animals live longer, it hardly promises a reasonable approach for humans. Even if promised extended longevity, few people would willingly cut back on calories by 40 percent.
Instead of eating less, perhaps humans can live longer through mechanisms related to the insulin-like signaling system, a complex hormonal pathway that plays a vital role in regulating an organism's energy allocations. It is known that inactivating parts of the insulin-like signaling system through mutations extends life and delays senescence in flies, mice and worms. The operative molecule controlled by this system is the insulin-like growth factor-1 (IGF-1), a signaling protein whose name derives from the fact that the gene and protein have a significant sequence homology with insulin. However, this protein's functions are quite distinct from insulin's.
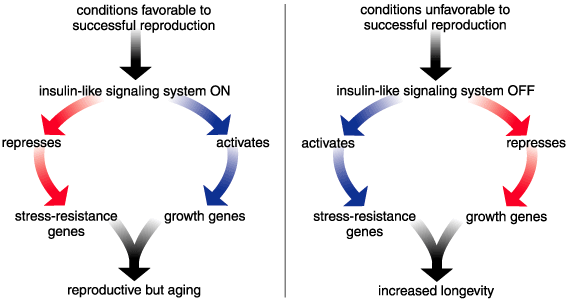
Apparently, the insulin-like signaling system can shift an organism's emphasis from growth to repair by modulating the IGF-1 level so as to activate or depress two diametrically opposed sets of genes. The growth set includes genes that bring about rapid body growth and a high reproductive rate. The stress-resistance set includes genes that make products that help an organism fight stress.
Under ordinary circumstances, it is a young organism's environment, including its diet, that controls the insulin-like signaling system. Conditions favorable for reproduction indirectly cause the release of high levels of IGF-1, which activates the insulin-like signaling system. Then, the insulin-like signaling system represses the stress-resistance genes and activates the growth genes. Unfavorable conditions, on the other hand, indirectly lead to a low level of IGF-1 and repress the insulin-like signaling system, which activates the stress-resistance genes and represses the growth genes. So, switching metabolism from a pro-growth stance to a pro-repair stance by somehow repressing the insulin-like signaling system does delay the onset of senescence in many organisms, including mammals. This, of course, is exactly what Martin Holzenberger and his colleagues did in the experiment described above when they decreased the number of IGF-1 receptors by 50 percent and thus lowered the effective concentration of the signaling molecules.
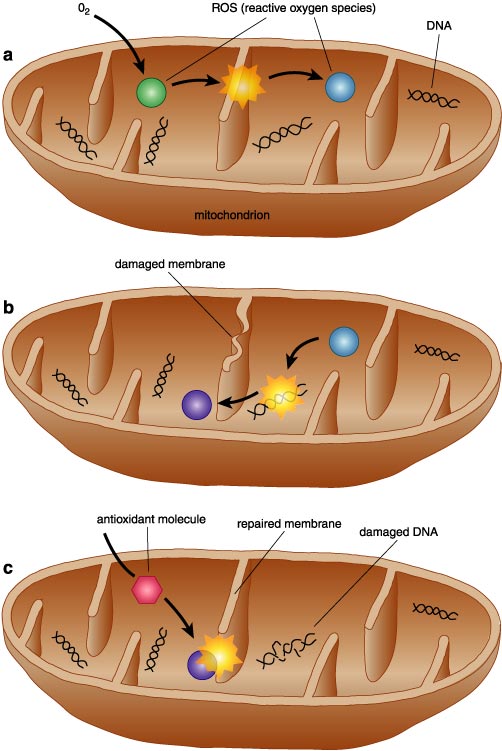
Without oxygen, we cannot generate enough energy to live, and we quickly die. Nonetheless, oxygen also breaks down in cells to yield ROS molecules. These reactive molecules combine with cellular components and transform them into oxygen-based damage products. This structural transformation alters a molecule's function, usually for the worse, and the damage process also generates another ROS molecule. The destruction rampages through a cell until an antioxidant molecule stops the damage. In so-called oxidative stress, a cell essentially goes through self-perpetuating rusting.
Once breathed in, oxygen goes to mitochondria to help convert the energy from food to a chemical form that is useful to cells. Depending on energy needs, a cell might have from ten to several hundred mitochondria. With most of a cell's oxygen located in mitochondria, that is where most of a cell's ROS get generated. A mitochondrion's lipid membrane and protein enzymes serve as the nearest target for the ROS, but these components can be repaired. Mitochondria, though, are the only animal-cell organelles with their own genetic system, and the one to ten mitochondrial DNA molecules are very vulnerable to irreparable oxidative damage. Injured mitochondria soon become almost nonfunctional. The resulting energy shortage inhibits a cell's normal functioning, and tissues start aging.
Tom Dunne
Organisms can fight off oxidative stress, and young animals eliminate most, but not all, of the ROS molecules. Sooner or later, inefficiencies appear in an organism's defense mechanisms. Then, the rate of cell damage compounds, and the age-related loss of function soon becomes apparent. In studies of flies, mice and worms, aging proceeded faster than ever, after inactivating oxidative stress–resistance genes. Apparently, stress-resistance pathways function in a parallel but integrated manner with the insulin-like signaling system. This mitochondrial–free radical theory of aging explains much of what happens in aging laboratory model systems and in humans.
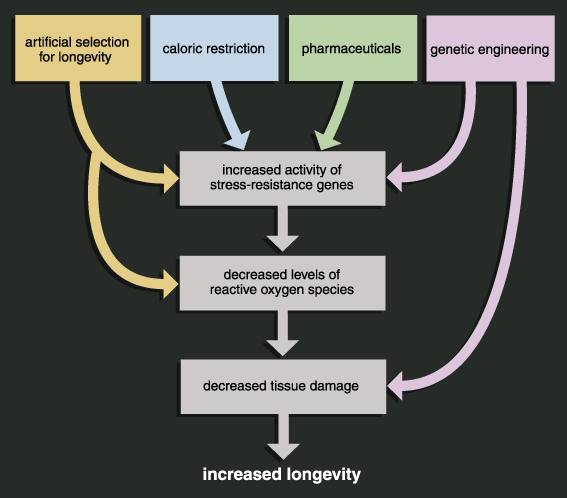
Many investigators realized that increasing the level of defense mechanisms against oxidative stress could extend an organism's health span. For example, several labs used genetic engineering to introduce extra copies of ROS-scavenging genes into otherwise normal flies, and the flies entered senescence later and lived longer, much like the artificially selected long-lived flies described above. Likewise, David Chavous of Boston College and his colleagues achieved similar results through the over expression of repair genes in flies, whose products repaired some proteins damaged by ROS. Working on worms, Pamela Larsen of the University of Southern California showed that up-regulating oxidative stress–resistance genes delayed the onset of senescence and created longer-lived animals; while Shuji Honda and Mitsuyoshi Matsuo of the Tokyo Metropolitan Institute of Gerontology demonstrated that higher-than-normal levels of oxygen accelerated the aging of both wild type and mutant animals.
My research group's work on artificial selection in flies also produced organisms with a much higher level of oxidative-stress resistance and more efficient mitochondria. In fact, the lower level of oxidative damage and delayed onset of senescence in those flies arose from decreased production and increased destruction of ROS. However, using genetic-engineering techniques to insert extra copies of these same oxidative stress–resistance genes into mice has not yet resulted in extending longevity.
Although the genetic manipulations explored in the lab do not make likely therapeutic tools so far, the results encouraged many scientists to explore pharmaceutical attacks on aging. A variety of such experiments recorded significant increases in an animal's health span or a significant extension of an animal's functional abilities. For example, Semour Benzer and his team at the California Institute of Technology fed fruit flies a drug called 4-phenylbutyrate, which inhibits enzymes used by the cell to repress its stress-resistance genes, and it delayed the onset of senescence. Nonetheless, different strains of flies needed different drug doses in order to yield the same result. This implies the existence of genetically based individual differences in response to drug-based interventions to increase longevity.
Tom Dunne
Other types of pharmaceutical interventions are also being pursued. For instance, Simon Melov of the Buck Institute for Age Research and his colleagues gave worms drugs which that functioned as synthetic superoxide dismutase/catalase enzymes, or mimetics, and scavenge excess ROS within cells. The worms lived 44 percent longer, on average. So pharmaceutical interventions against aging seem feasible.
Nonetheless, people should mistrust the nonscientific claims and blarney put out by the present antiaging industry. For example, at least 250,000 Web sites sell human growth hormone, and many tout it as a cure for aging. It is not. In the original study behind antiaging claims for growth hormone, a dozen men showed positive effects at first, but then suffered deleterious side effects that cancelled the study. Now, scientific data suggest that taking growth hormone advances aging—quite the opposite from what the ads purport.
Tom Dunne
The future of aging research faces three significant questions. First, can science increase the health span of a laboratory primate? Second, will similar interventions extend human life in a safe way? Third, will public debate on this matter encourage or inhibit using this knowledge? Given the success of pharmaceuticals extending the health span of invertebrates, a similar outcome seems biologically reasonable in mammals, but proving that will take some time—definitely years, although no one knows how many. In monkeys, perhaps another decade will provide good data on whether interventions can slow the rate of aging, but it could take longer for a complete assessment of lifetime effects. The pace of discovery in this field, however, increases rapidly, so these time lines might be too conservative.
If someone finds a pharmaceutical that mimics the effects of caloric restriction or of IGF-1 reduction or of releasing the repression of stress-resistance genes, it might be possible to add about 25 years to a person's lifespan. Humans might then be healthy adults from the age of 20 to 80 years, instead of the current 20 to 55 or so. That sounds great to most people, but some critics see only increased despair and financial costs. This criticism, however, overlooks the fact that the senescent phase will stay the same in absolute terms, and the associated costs will not change. In fact, an increased health span will not cost more. Instead, it would give us longer, healthier and more productive lives.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.