Zika Goes Viral
By Robert Dorit
While the Zika virus has its moment, few people are discussing the problems underlying the worldwide increase in emerging infectious diseases.
While the Zika virus has its moment, few people are discussing the problems underlying the worldwide increase in emerging infectious diseases.

DOI: 10.1511/2016.122.274
Most epidemics begin quietly. Beginning in 2015, health workers in Brazil noted an increase in the number of cases of a relatively mild and nondescript infection, likely caused by a mosquito-borne virus known as Zika. On May 7, 2015, the Pan American Health Organization and the World Health Organization (WHO) issued an epidemiological alert that calmly stated, “Currently, the public health authorities of Brazil are investigating a possible transmission of the Zika virus in the northeast of the country.” This outbreak seemed little more than one more skirmish in our ongoing war with emerging infectious diseases.
By the fall of 2015, however, scientists began to realize that the Zika outbreak was more than a small-stakes epidemic. This viral outbreak was one with potentially horrific consequences, as children born with abnormally small heads suddenly began to appear with alarming frequency in the northeast provinces of Brazil—almost exactly 38 weeks after reported increases in the incidence of Zika infections. In the Brazilian state of Bahia, the risk of giving birth to a microcephalic child had gone from a background rate of 0.02 percent to anywhere between 0.88 and 13.2 percent for women infected with the Zika virus in the first trimester of pregnancy.
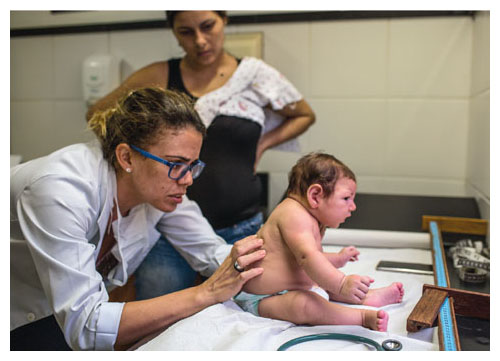
Photograph by Lianne Milton
As I write this, more than 406,755 suspected cases of the Zika infection have been reported in the Americas, and 56,685 of them have been confirmed. This virus, until recently the largely ignored and underachieving cousin of the far better-known Dengue fever, yellow fever, and West Nile fever viruses, has suddenly gained a terrible prominence. In the wake of this disease, thousands of devastated parents now care for newborns with severe neurological damage.
Yet Zika will not necessarily remain in the spotlight for long. An argument can be made that epidemics are now subject to the Warhol Effect—every infectious agent is bound to have its 15 minutes of fame. Our short attention spans, as well as our thirst for drama and the amplifying effects of modern media, conspire to make every emerging disease a short-term cause célèbre that is forgotten all too quickly.
For those of us who spend our professional lives thinking about infectious disease, the Warhol Effect has profound implications: The thousands of cases of Zika recorded to date draw public attention (and funding) away from prevalent, deadly diseases that people have simply grown weary of hearing about. Some 214 million cases of malaria were reported in 2015. Taken together, tuberculosis, HIV, malaria, diarrheal disease, and pneumonia kill one in five people worldwide, but they are no longer news. For the unaffected, these diseases have become part of a mundane, if grim, backdrop. If the problems underlying all these diseases were better addressed, lives could be saved.
Nevertheless, Zika does deserve attention. Not only is the human cost of this disease profound and, for many, lifelong, this outbreak reveals new weaknesses and strengths in our ongoing standoff with infectious agents. At the same time, this outbreak underscores the importance of viewing ourselves as global citizens. At a time when globalization is viewed with increasing distrust and transnational cooperation is portrayed as inimical to national interests, outbreaks remind us that we are in this together. This Zika outbreak is important in and of itself, but it is also part of a larger pattern that is sometimes hidden. Increasingly frequent disease outbreaks around the globe require a concerted response. Rather than careening from crisis to crisis, closing borders, and blaming migrants, we need ongoing support for international efforts and institutions that monitor and respond coherently to infectious disease.
Following its identification in 1947, Zika had only occasionally been detected in Africa (or elsewhere): Fewer than 20 cases were reported before 1981. The actual number of cases was certainly higher, but a virus that causes mild and fairly generic clinical symptoms is likely to be ignored and its numbers underestimated. More detailed and focused surveys of selected populations in Africa reveal the presence of anti-Zika antibodies in 38 percent of individuals sampled, suggesting widespread undiagnosed and possibly asymptomatic exposure to it.
The virus spread while we, the host, barely noticed. That changed in the following decades. In 2007 on the tiny Micronesian island of Yap, 108 cases of Zika infection were reported, and 73 percent of residents above the age of three were exposed to the virus. In 2013 the virus struck French Polynesia, affecting as many as 32,000 people. A year later a visitor, likely coming from the Pacific islands to Brazil, acted as an unsuspecting courier for a virus not previously seen in the Americas.
This outbreak is still in its early phase; the epidemic has not yet peaked. The virus has spread far from its original epicenter in northeast Brazil, moving southward into other South American countries and inexorably northward toward the United States. Since 2015, 47 countries have reported Zika transmission for the first time. The magnitude of this event begs for an explanation. What is different this time? What changes have turned this unprepossessing virus into a global villain?
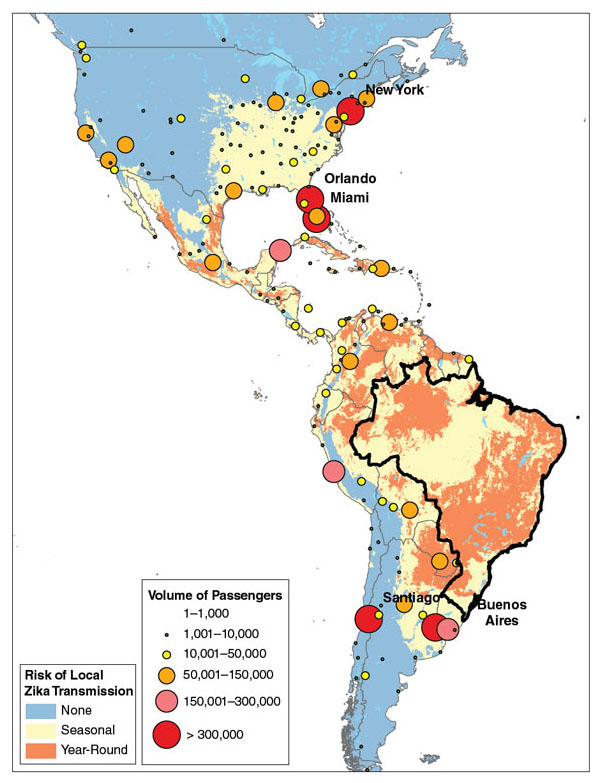
Courtesy of Kamran Khan, St. Michael’s Hospital, Toronto, Canada.
The answers are not straightforward. Epidemiology has its troika—host, agent, and vector—and all three are in motion. Over the past two decades, the human population has unwittingly reconfigured itself to optimize the spread of infectious diseases. The global population continues to grow at more than 1.1 percent per year, expanding the pool of susceptible hosts. More than half of the human population now lives in cities, increasing the probability of transmission for any communicable disease. Every day, on average, more than 8 million people are in the air, and millions more are on the move, transporting potential pathogens quickly around the globe. A more propitious set of conditions for epidemic spread would be hard to imagine. To date, 934 possible cases of Zika in the United States can be traced directly to travelers arriving from countries with a high incidence of the infection.
But these trends are not new, nor are they specific to Zika. Although increasing population and travel certainly account, at least in part, for the increasing frequency of emerging infectious diseases, these variables alone cannot explain this outbreak. The virus, too, is evolving rapidly as it adapts to changing circumstances. Like other viruses that preserve their genetic information in the form of RNA, it has made constant change its calling card. In these RNA viruses, the machinery responsible for copying and transmitting genetic information from one generation to the next is notoriously inaccurate.
In contrast to species that have evolved painstaking fidelity when replicating their genomes, RNA viruses pass on to the next generation genetic information riddled with mistakes, many costly. But with such sloppiness comes the promise of innovation: Every viral particle, slightly different from its parent, is an evolutionary experiment. A virus and its offspring are not so much a close-knit family as a swarm of related particles exploring the environment. The strategy has proven fiendishly effective for many viruses, allowing them to evolve rapidly in response to the biology of their hosts and vectors. Not surprisingly, the original Zika strain has diversified into hundreds of distinct isolates whose history records the inexorable march of this virus across the globe.
We cannot yet pinpoint the exact changes in the viral genome that have transformed the comparatively benign Zika virus of the 1950s into the aggressive one of the 2010s. In the thousands of generations that have elapsed since its discovery, this virus has refined its ability to home in on neural stem cells—the progenitors of the fetal brain—by exploiting an abundant receptor on the cell surface to enter into and subsequently hijack the machinery of these cells. The Zika virus has also evolved the capacity to cloak itself in maternal antibodies to cross the placental barrier during pregnancy.
We are not yet certain about what precise information encoded in the Zika genome makes such acts of subterfuge possible, but a suite of mutations holds a partial answer. More recently, the possibility that Zika can be sexually transmitted has come into focus. The case for this route of transmission is still largely circumstantial. A small number of Zika cases in individuals who do not live and had not travelled to areas where the virus and its mosquito vector are present could be explained only by sexual contact with a partner who had recently travelled to a Zika-endemic region.
Furthermore, significant concentrations of active Zika virus have now been found in the seminal fluid of infected men. The virus is exploiting the privileged status of the male reproductive tract, where immune scrutiny is reduced to ensure the survival of sperm cells. By congregating in this protected region, the virus may be evolving a new route of transmission and in the process freeing itself from dependence on a mosquito vector. It is too early to say just how significant this new mode of transmission will be. But for the most part, pathogens, and especially viruses, that have hitched their fate to the persistence and prevalence of sexual intercourse have done chillingly well.
Still, the startling evolvability of this virus cannot fully explain the causes of the current epidemic. The last member of the troika, the mosquito, is also in the midst of a dramatic transformation. Mosquitoes are the bush pilots of infectious disease. They transport all manner of pathogens from one host to another, often oblivious of their cargo. Mosquitoes seem to derive little evolutionary profit from this dirty job: The pathogens have simply evolved to hijack this efficient route of transmission. Unbeknownst to the mosquito drawing the blood meal from its host, it is also a transient but indispensable refuge for bloodborne pathogens. The Zika virus, unceremoniously ingested in the course of a normal feeding, migrates over the next 10 days from the mosquito’s gut into its circulatory system and eventually to its salivary glands, ready to be injected into a new host as soon as the mosquito bites again.
Two invasive species of mosquito, Aedes aegypti and Aedes albopictus, appear to be primarily responsible for the spread of this virus in the Americas. These two species differ in their ecology and habits. A. aegypti is primarily diurnal, feeds outdoors, and ventures far from its birthplace. In contrast, A. albopictus feeds indoors, primarily in the mornings and evenings. Together, they have evolved to take full advantage of ecological opportunities provided by human habitation and can feed on us the entire time we are awake. These mosquitoes are also sip feeders: Preferring more tapas than sit-down meals, they will feed on multiple hosts, taking only small sips of blood from each of them. Each of these meals, in turn, facilitates the spread of the virus to multiple hosts.
Epidemiology is not for the faint of heart. Public health authorities must strike a balance between premature action and overly cautious delay.
The Zika virus exploits the vectors’ ecology to increase its transmission. Perhaps most alarming of all, the geographic range of both mosquito species is rapidly expanding. Driven by a combination of increased urbanization, poor sanitation, and climate change, these mosquitoes are on the move. Confined to West Africa until the 15th century, and more recently found mainly in a tropical band around the equator, A. aegypti has now established a beachhead as far north as Virginia. Over the past 75 years, A. albopictus has spread from its original range in the South Asian subcontinent to the New World, where it is found from Patagonia to Massachusetts. The risk of Zika infections moves with the mosquitoes. In the continental United States the prospect of Zika infections has gone from prediction to reality in the past months. In response to the epidemic, much attention has focused on the eradication of mosquitoes in areas where Zika has gained a foothold, as well as in regions where densities of potential vectors are high.
Although conventional approaches to mosquito eradication, including draining bodies of standing water and using insecticides, have proven somewhat effective in reducing mosquito populations, complete eradication by these means appears unrealistic. Both mosquitoes have adapted to the opportunities provided by human habits, laying their eggs in even the tiniest pool of standing water. Ambitious projects use the latest genetic tools to create mosquitoes that sow the seeds of their own destruction or to deliberately infect mosquitoes with Wolbachia, a bacterium that prevents Zika from colonizing the mosquito. Vaccines against Zika have been developed in record time and are now in clinical trials. Taken together, these breakthroughs will help people regain the upper hand, but such developments will take time to come into use. Our collective immunity and ingenuity will eventually make the virus recede, for now, back into its wildlife reservoir. But the human population will still remain ripe for future infections.
How could we not have seen this, or other important outbreaks of the past several decades, coming? After all, the infectious agents responsible for recent outbreaks were for the most part already known. The factors underlying epidemics—population density, poverty, globalization—have long been identified and are increasingly well understood. Yet we seem constantly caught unaware, unable to predict, and slow to respond. What aren’t we seeing?
Part of the answer requires recognition that epidemiology is high-stakes science. All scientists are trained to be cautious in the interpretation of data, circumspect in any extrapolation, and careful not to let conclusions outpace evidence. These practices underlie the resounding successes of science but require persistence, support, and, above all, time. Public health authorities responding to an outbreak do not have the luxury of waiting until all the facts are in. Instead, they must strike a balance between premature action and overly cautious delay. Respond too quickly, and resources are squandered for no reason. Respond too cautiously, and infections and deaths that could have been prevented aren’t. Epidemiology is not for the faint of heart.
On February 1 of this year, just weeks after the rise in microcephaly cases in Brazil had come into focus, the WHO declared Zika a public health emergency of international concern. This official designation had important regulatory, fiscal, and policy consequences and was intended to focus the attention and efforts of the international community on this emerging threat. The WHO had chosen to sound the alarm well before the scope of the outbreak was understood and before the link between Zika and neurological defects was clear. Yet the costs of waiting for definitive proof were simply too high: The caution and conservatism inculcated into every scientist had to be tempered with the dangers of delay. Their decision was sound in part because in this field proof means something very particular.
Events associated in time and space are the currency of epidemiology. Proof in this field, for ethical and practical reasons, seldom comes from direct experimentation. Under such circumstances correlation here is indirect evidence of causation. Proof that Zika causes microcephaly will not be definitively established in the conventional sense for quite some time, although evidence for the link is mounting. The molecular, cellular, and developmental evidence relating the virus to its devastating consequences will take painstaking work to accumulate. Until then, the power of statistical inference needs to be brought to bear: Epidemiologists could not explain the coupled rise of Zika and microcephaly except by arguing, at least for now, that the virus is the cause of the neurological defects.
Epidemiology challenges the misconception that the capacity for exact prediction is the hallmark of any real science. Like cosmology and evolutionary biology, epidemiology operates in a realm where history and contingency play fundamental roles. To be sure, this field strives to be predictive: Epidemiologists try to anticipate the severity of an infectious outbreak, pinpoint the location of the next emergent disease, and gauge the potential impact of public health interventions. We do so by using the past as a way to anticipate the future. But extrapolation is a dodgy game when dealing with phenomena involving so many components.
Every player in a disease outbreak—host, vector, reservoir, infectious agents—is constantly changing. No two outbreaks can be exactly the same. The Zika virus, first isolated from a rhesus monkey in Uganda in 1947, has evolved dramatically since then. The two previous Zika outbreaks (on Yap and in French Polynesia) differed from the current one in their severity and consequences. Every outbreak results from a confluence of events that will never be repeated. Epidemiology’s power to predict in detail is thus necessarily constrained. In exchange, epidemiologists mine these unique events for their regularities, deepening our understanding of the ongoing dance between humans and their pathogens.
People are again locked in combat with an emergent disease that, at least for now, appears to have the upper hand. These early skirmishes have revealed a virus capable of exploiting every opportunity we have provided. The consequences of a Zika infection vary considerably: for some, just a slight fever and rash; for others, temporary paralysis and other severe neurological effects; and for pregnant women, the prospect of a microcephalic newborn. It seems cruel to imagine that these are all incidental consequences of a virus shaped by evolution.
The struggle between pathogens and hosts is as old as life itself and has resulted in an unending series of fragile truces. In that sense, the Zika outbreak is just one more in a long line of engagements. But such struggles are becoming more common: New infectious diseases are emerging (or reemerging) more frequently than ever before. Something is changing in our relationship to pathogens. Throughout human history, most infectious disease outbreaks were local. Concentrated in one or at most a few small human populations, infections would quickly run their course, leaving only death and immune survivors in their wake. All that has changed now.
As hosts, vectors, and pathogens move quickly around the globe, we would do better to imagine the human population as a single body. Infection in any part of this body politic threatens the whole. Likewise, an effective response to this new reality transforms our surveillance, containment, and treatment efforts into a planetwide immune response. In a globalized world, eternal vigilance is the price of health.
Daunting as the problem of emergent infections might seem, we are far from powerless before it. New technologies, from mobile phones to Internet searches, now serve as early warning systems for outbreaks. Novel diagnostic technologies make it possible for epidemiologists to pinpoint infectious agents more quickly and with greater resolution than ever before. The very interconnectedness that helps spread infection also serves to recruit citizens and experts in efforts to monitor the earliest stages of an outbreak. The earlier we detect one, the better is the chance of containment. Through our cooperative community-level and international efforts, we become more than passive victims. We are now the resistance.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.