How Can You Mend a Broken Heart?
By Maggie DeMonia
Reverting cardiac cells to an infant state could fix tissue damage from a heart attack.
July 30, 2024
From The Staff Biology Medicine Anatomy Cytology Physiology
In the time it will take to read this article, at least two people in the United States will experience a heart attack. Although some patients recover, all survivors from a heart attack, also known as myocardial infarction, are left with damaged heart tissue that will never heal. As the rate of cardiovascular disease diagnoses continues to rise, the threat of irreversible damage from a heart attack looms for an increasing number of people. But a group of scientists in Germany may have uncovered a possible key to healing damaged hearts, and it lies in the energy we get from our food.
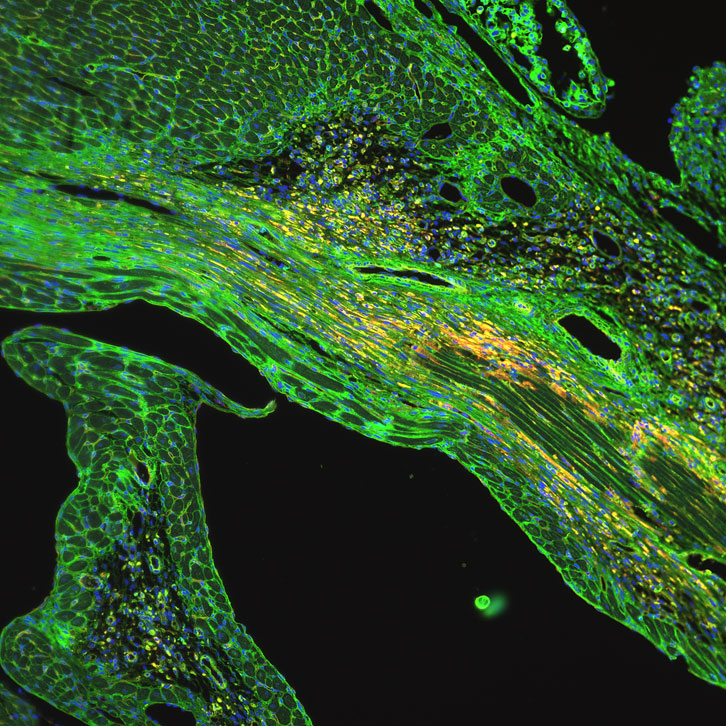
Image by Daria Monogiou Belik, University of Basel, CC BY-NC-ND 2.0
Metabolism, a series of chemical reactions that occurs in every cell of the body, converts nutrients into energy that cells can use to perform a variety of biological functions. And like the food it processes, metabolism can come in many different flavors, which depend on the cell type, its stage of development, and its condition. The cells of a fetus that divide and expand to grow into a new heart use a type of metabolism called glycolysis, which involves the splitting of sugar molecules. But after birth, the cells responsible for the muscle contraction and general function of the heart, called cardiomyocytes, shift from glycolysis to a process called fatty acid oxidation, in which lipid molecules essentially are broken apart to release energy. Once this change in metabolism occurs, cardiomyocytes lose the ability to divide and generate new heart tissue, leaving no method for repair if that tissue is damaged.
There may be a way for cardiomyocytes to return to their youth, however. Thomas Braun and A. Xuejun Yuan of the Max Planck Institute for Heart and Lung Research, along with their colleagues, recently reported in the journal Nature that when fatty acid oxidation is blocked in the cardiomyocytes of adult mice, the cells revert to the glycolysis they used before birth. With this change in metabolism, cardiomyocytes also regained the ability to regenerate and grow new heart tissue.
To create this metabolic change in mice, Braun and Yuan’s team turned to genetics. They found that a gene called Cpt1b, which is associated with fatty acid oxidation, is increasingly active as cardiomyocytes mature and switch from glycolysis to fatty acid oxidation after birth. Following damage to mouse heart muscle, like that which occurs during a heart attack, the researchers deleted the Cpt1b gene from the cardiomyocytes, triggering a shift from fatty acid oxidation back to glycolysis. As a result, the mice showed signs of cardiac tissue regeneration and significant restoration of heart function, similar to what seen before the injury.
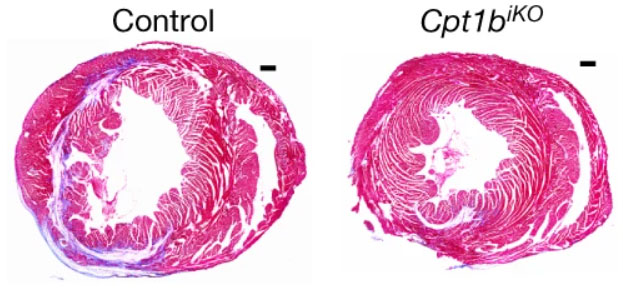
Image from X. Li et al, 2023, Nature 622:619.
The group then investigated how this change in metabolism alters heart regeneration. “We really wanted to understand the underlying mechanism,” says Braun. “When you block fatty acid oxidation, why is it that the cardiomyocytes are now able to divide?” The answer was alpha-ketoglutarate, a member of a family of molecules known as keto acids that are important in metabolic processes. But alpha-ketoglutarate has a second job in the regulation and function of genes, including those that affect the ability of cardiomyocytes to mature and divide. These dual roles identify alpha-ketoglutarate as the link between cell metabolism and regeneration.
Although this study showed a promising treatment for mice, it’s a long way from a pharmaceutical option for human treatment. Drugs that inhibit Cpt1b exist, but similar enzymes, Cpt1a and Cpt1c, are also affected by these medications. As Braun recounts, “We tried that [and saw] a lot of liver damage. We couldn’t even do any regeneration experiments because the mice died rather quickly. So the challenge is to develop inhibitors that only inhibit Cpt1b.”
But new drugs are being discovered at an ever-increasing speed, so it may not be long until hypothetical Cpt1b-specific inhibitors become a reality for patients recovering from heart attacks. Further, Cpt1b has limited expression in other tissues, which leads Braun to believe that these potential drugs could be effective without harmful side effects. “This,” says Braun, “gives me a bit of optimism and hope.”
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.